Your How to write grams per mole images are ready in this website. How to write grams per mole are a topic that is being searched for and liked by netizens today. You can Find and Download the How to write grams per mole files here. Find and Download all royalty-free images.
If you’re looking for how to write grams per mole pictures information related to the how to write grams per mole topic, you have pay a visit to the right blog. Our website always provides you with hints for seeking the maximum quality video and picture content, please kindly hunt and locate more enlightening video articles and graphics that match your interests.
How To Write Grams Per Mole. The unit for atomic masses is number of grams per mole or gmol. The exact value of one mole is 6022 140 78 1023. One mnemonic device for. First the grams of the entire solution reagent solvent are found by multiplying volume by the density of the solution.
 How To Convert Grams To Moles Video Lesson Transcript Study Com From study.com
How To Convert Grams To Moles Video Lesson Transcript Study Com From study.com
35453 x 2 70096 grams per mole. Mole is the SI unit used to measure how many molecules or atoms there are. There is only 1 atom per element so we multiply the atomic masses by 1. 1 mole is equal to 1 moles In or 114818 grams. First identify the amount of grams that are in the problem. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula.
If 30 by mass multiply by 030.
Next the grams of reagent are found my multiplying the total grams by the percent reagent as a decimal ex. Next the grams of reagent are found my multiplying the total grams by the percent reagent as a decimal ex. For H 2. Molecular weight of In or grams The SI base unit for amount of substance is the mole. First identify the amount of grams that are in the problem. So you have 772830 258 moles.
 Source: pinterest.com
Source: pinterest.com
Moles to Grams Conversion Formula Questions. Salinity is usually expressed as parts per thousand ppt. Kilogram per mole kgmol 10 -3. First find out how many moles of C2H6 that you have. This chemistry video tutorial explains how to convert the unit grams to moles which is a common conversion step for many stoichiometry questions.
 Source: tr.pinterest.com
Source: tr.pinterest.com
Kilogram per mole kgmol 10 -3. If 30 by mass multiply by 030. First the grams of the entire solution reagent solvent are found by multiplying volume by the density of the solution. We assume you are converting between moles In and gram. Next the grams of reagent are found my multiplying the total grams by the percent reagent as a decimal ex.
 Source: pinterest.com
Source: pinterest.com
Where is the molar mass of the substance. The unit is typically gmol. In order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass. The mole is the SI unit of the measurement for the amount of a substance. Do a quick conversion.
 Source: pinterest.com
Source: pinterest.com
Mole is the SI unit used to measure how many molecules or atoms there are. First find out how many moles of C2H6 that you have. The following steps are how to convert grams to moles. 1 grams per mole 0001 grams per millimole using the online calculator for metric conversions. Mole is the SI unit used to measure how many molecules or atoms there are.
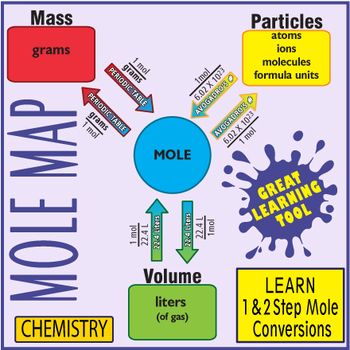 Source: pinterest.com
Source: pinterest.com
And for Cl 2. We assume you are converting between moles In and gram. The exact value of one mole is 6022 140 78 1023. 159994 x 2 319988 grams per mole. First identify the amount of grams that are in the problem.
 Source: pinterest.com
Source: pinterest.com
Kilogram per mole kgmol 10 -3. 1 grams per mole 0001 grams per millimole using the online calculator for metric conversions. 1 mole is equal to 1 moles In or 114818 grams. First the grams of the entire solution reagent solvent are found by multiplying volume by the density of the solution. The mole is the SI unit of the measurement for the amount of a substance.
 Source: pinterest.com
Source: pinterest.com
There is only 1 atom per element so we multiply the atomic masses by 1. In order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass. But wait what actually is a mole. N m M where M is the molar mass of this material. There is only 1 atom per element so we multiply the atomic masses by 1.
 Source: pinterest.com
Source: pinterest.com
Salinity grams total solutes per kg of seawater. Mole is the SI unit used to measure how many molecules or atoms there are. The unit is typically gmol. And for Cl 2. More commonly written for this application as.
 Source: pinterest.com
Source: pinterest.com
N m M where M is the molar mass of this material. 1 grams per mole 0001 grams per millimole using the online calculator for metric conversions. But wait what actually is a mole. Molecular weight of In or grams The SI base unit for amount of substance is the mole. Scientists use this number because 1 gram of hydrogen is around 1 mole of atoms.
 Source: no.pinterest.com
Source: no.pinterest.com
If 30 by mass multiply by 030. But wait what actually is a mole. More commonly written for this application as. Where is the molar mass of the substance. Salinity grams total solutes per kg of seawater.
 Source: youtube.com
Source: youtube.com
First find out how many moles of C2H6 that you have. First the grams of the entire solution reagent solvent are found by multiplying volume by the density of the solution. Moles to Grams Conversion Formula Questions. The unit is typically gmol. Salinity grams total solutes per kg of seawater.
 Source: youtube.com
Source: youtube.com
Gram per mole gmol 1. 1 mole is equal to 1 moles In or 114818 grams. We assume you are converting between moles In and gram. Then find the substances molar mass. Gram per mole gmol 1.
 Source: pinterest.com
Source: pinterest.com
If 30 by mass multiply by 030. For H 2. We assume you are converting between moles In and gram. Gram per mole gmol 1. Then find the substances molar mass.
 Source: wikihow.com
Source: wikihow.com
Next the grams of reagent are found my multiplying the total grams by the percent reagent as a decimal ex. The following steps are how to convert grams to moles. If 30 by mass multiply by 030. Where is the molar mass of the substance. Moles to Grams Conversion Formula Questions.
 Source: study.com
Source: study.com
The unit is typically gmol. 1 mole is equal to 1 moles In or 114818 grams. Then find the substances molar mass. In order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass. Lastly divide the grams from the first step by the molar mass in the second step to find the answer.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to write grams per mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






