Your How to work out the molecular weight of a compound images are ready. How to work out the molecular weight of a compound are a topic that is being searched for and liked by netizens now. You can Find and Download the How to work out the molecular weight of a compound files here. Download all free photos.
If you’re searching for how to work out the molecular weight of a compound pictures information connected with to the how to work out the molecular weight of a compound topic, you have come to the right blog. Our website frequently provides you with hints for viewing the maximum quality video and picture content, please kindly surf and find more informative video articles and images that fit your interests.
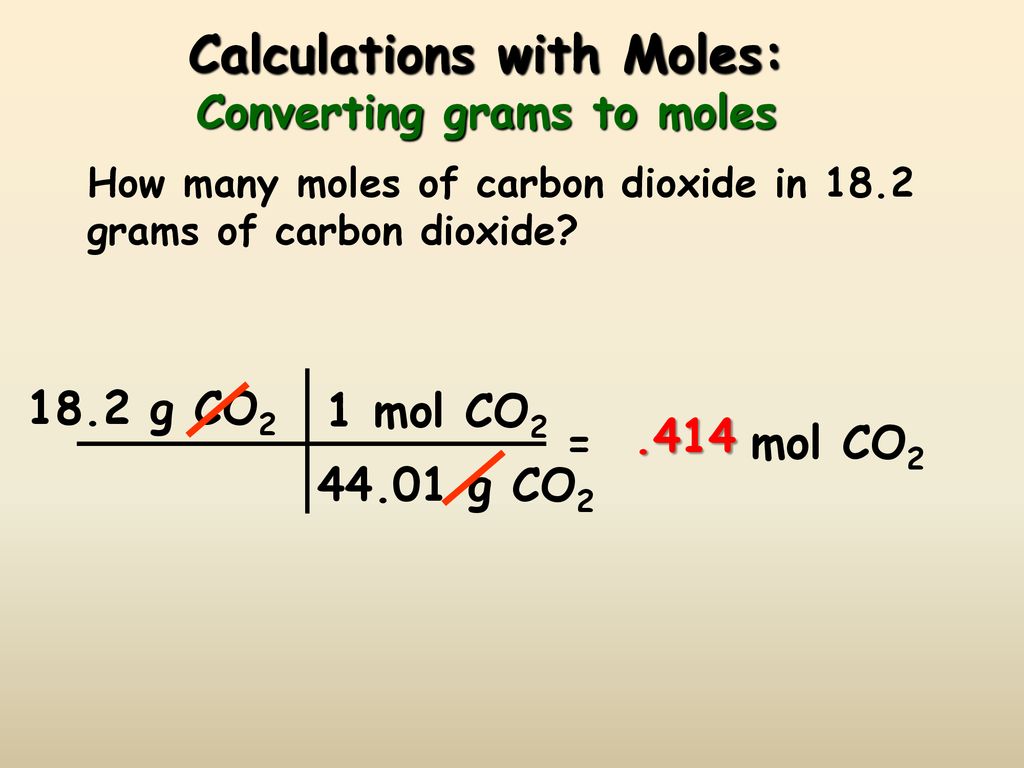
How To Work Out The Molecular Weight Of A Compound. The molecular weight of the compound determines the mass of 1 mole of the specific substance and the number of grams per mole of the compound. The molar mass was given as 60 gmol. The answer of 19713 mg appears in the Mass box. Please remember that you need the molar mass first when trying to find the average mass of one molecule.
 How To Calculate A Molecular Formula Molecular Chemistry Class Molar Mass From pinterest.com
How To Calculate A Molecular Formula Molecular Chemistry Class Molar Mass From pinterest.com
Thus if the value of the former is 2 then the molecular formula suffixes are twice of the ones present in the empirical formula. Cu 1 x 6355 6355. The molecular weight of this compound is 18018gmol. 2 of hydrogen H and 1 oxygen O atom. S 1 x 3207 3207. The molar mass of water is 18015 gmol.
You can use that more accurate information about the mass of the molecular ion to work out the molecular formula of the compound.
So far weve been looking at mz values in a mass spectrum as whole numbers but its possible to get far more accurate results using a high resolution mass spectrometer. Molecular weight can be measured in many ways but UV spectrophotometry is not one of them. Enter 19713 into the Molecular Weight MW box. Cu 1 x 6355 6355. Next you add these two numbers together to get 5844 gmol of NaCl. Divide the molar mass for the molecular formula by the empirical formula mass.
 Source: pinterest.com
Source: pinterest.com
Molecular weight MW is the weight of one mole of a chemical. The result determines how many times to multiply the subscripts in the empirical formula to get the molecular formula. Thus if the value of the former is 2 then the molecular formula suffixes are twice of the ones present in the empirical formula. The mass of the atoms in the empirical formula is 14. M r of NaOH 23 16 1 40.
 Source: pinterest.com
Source: pinterest.com
Molecular weight MW is the weight of one mole of a chemical. To find the molar mass of sodium chloride you first go to the periodic table. In order to calculate the molecular weight of one water molecule we add the contributions from each atom. The number of empirical units correspond to the molecular units. Molecular mass 40078 x 3 3097361 x 2 159994 x 8 molecular mass 120234 6194722 1279952 molecular mass 31017642 from the calculator molecular mass.
 Source: pinterest.com
Source: pinterest.com
Mass g Concentration molL x Volume L x Molecular Weight gmol volume of a solution calculation. The chemical formula for water is H2O which means this molecule has 3 atoms. The number of empirical units correspond to the molecular units. From the equation 2 mol of. Molecular mass 40078 x 3 3097361 x 2 159994 x 8 molecular mass 120234 6194722 1279952 molecular mass 31017642 from the calculator molecular mass.
 Source: pinterest.com
Source: pinterest.com
The mass of the atoms in the empirical formula is 14. From the equation 2 mol of. To find the molar mass of sodium chloride you first go to the periodic table. O 9 x 159994 1439946. Mass of a compound of a solution calculation.
 Source: pinterest.com
Source: pinterest.com
Then you can use methods such as osmolality freezing point. The molecular weight of this compound is 18018gmol. Empirical formula weight 1 x 1201gmol 2 x 101gmol 1 x 1600gmol 3002gmol. Number of moles of NaOH mass relative formula mass 20 40 05 mol. Volume L Mass g Concentration molL x Molecular Weight gmol molecular weight of a solvent in a solution calculation.
 Source: pinterest.com
Source: pinterest.com
Molar mass of CH 2. Ca 1 x 40078 40078. Add them up Molecular Weight249694gmol. In order to calculate the molecular weight of one water molecule we add the contributions from each atom. Molecular weight MW is the weight of one mole of a chemical.
 Source: pinterest.com
Source: pinterest.com
The molar mass of water is 18015 gmol. Thus if the value of the former is 2 then the molecular formula suffixes are twice of the ones present in the empirical formula. Using the periodic table of the elements to find atomic weights we find that hydrogen has an atomic weight of 1 and oxygens is 16. Therefore the unit of. M r of Na 2 SO 4 23 23 32 16 16 16 16 142.
 Source: pinterest.com
Source: pinterest.com
S 1 x 3207 3207. The number of empirical units correspond to the molecular units. Therefore the unit of. Then we should find out how much CH 2 O units are present there. Molecular weight can be measured in many ways but UV spectrophotometry is not one of them.
 Source: nl.pinterest.com
Source: nl.pinterest.com
Using a mass spectrum to find a molecular formula. The molecular weight of this compound is 18018gmol. This was calculated by multiplying the atomic weight of hydrogen 1008 by two and adding the result to the weight for one oxygen 15999. Add up the atomic masses of the atoms in the empirical formula. The result determines how many times to multiply the subscripts in the empirical formula to get the molecular formula.
 Source: pinterest.com
Source: pinterest.com
Number of moles of NaOH mass relative formula mass 20 40 05 mol. It helps immensely if your molecule is pure. Therefore the unit of. Add up the atomic masses of the atoms in the empirical formula. 2 of hydrogen H and 1 oxygen O atom.
 Source: pinterest.com
Source: pinterest.com
Cu 1 x 6355 6355. The molecular weight of the compound determines the mass of 1 mole of the specific substance and the number of grams per mole of the compound. Sample Molecular Weight Calculation. Number of moles of NaOH mass relative formula mass 20 40 05 mol. The result determines how many times to multiply the subscripts in the empirical formula to get the molecular formula.
 Source: br.pinterest.com
Source: br.pinterest.com
Or you can choose by one of the next two option-lists which contains a series of common organic compounds including their chemical formula and all the elements. Thus if the value of the former is 2 then the molecular formula suffixes are twice of the ones present in the empirical formula. Cu 1 x 6355 6355. The mass of the atoms in the empirical formula is 14. This online calculator you can use for computing the average molecular weight MW of molecules by entering the chemical formulas for example C3H4OHCOOH3.
 Source: pinterest.com
Source: pinterest.com
O 9 x 159994 1439946. In order to calculate the molecular weight of one water molecule we add the contributions from each atom. Then you can use methods such as osmolality freezing point. From the equation 2 mol of. Sample Molecular Weight Calculation.
 Source: in.pinterest.com
Source: in.pinterest.com
Then you can use methods such as osmolality freezing point. For sodium it is 2299 gmol while for chlorine it 3545 gmol. Molecular weight MW is the weight of one mole of a chemical. In other words the molar mass is the total mass in grams of all the atoms that make up one mole of a given molecule. Therefore the unit of.
 Source: pinterest.com
Source: pinterest.com
You can use that more accurate information about the mass of the molecular ion to work out the molecular formula of the compound. Therefore the unit of. The result determines how many times to multiply the subscripts in the empirical formula to get the molecular formula. From the equation 2 mol of. You can use that more accurate information about the mass of the molecular ion to work out the molecular formula of the compound.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to work out the molecular weight of a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.