Your How to turn molar mass into mass images are ready. How to turn molar mass into mass are a topic that is being searched for and liked by netizens now. You can Find and Download the How to turn molar mass into mass files here. Get all free photos.
If you’re searching for how to turn molar mass into mass pictures information linked to the how to turn molar mass into mass interest, you have pay a visit to the right blog. Our site always provides you with hints for seeking the highest quality video and image content, please kindly surf and locate more informative video content and graphics that fit your interests.
How To Turn Molar Mass Into Mass. Use the molecular formula to find the molar mass. Convert 02 moles of Sodium chloride. Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams. In that case you always have 60210 23 molecules in a mole Avogadros number so the molar mass divided by Avos number gives the mass of the molecule.
 Converting Between Moles Mass And Number Of Particles Number Of Particles To Mass Chemistry 11 Ms Mcgrath Ppt Download From slideplayer.com
Converting Between Moles Mass And Number Of Particles Number Of Particles To Mass Chemistry 11 Ms Mcgrath Ppt Download From slideplayer.com
Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams. Mass g No. The base SI unit for mass is the kilogram and that for amount of substance is the mole. How do you convert moles to molar mass. By dividing the total mass of the number of moles that you areconsidering by number of moles. ___g H2O x 1 mol H2O 180g H2O ___ mol H2O ___ mol H2O x 180g H2O 1 mol H2O ___ g H2O.
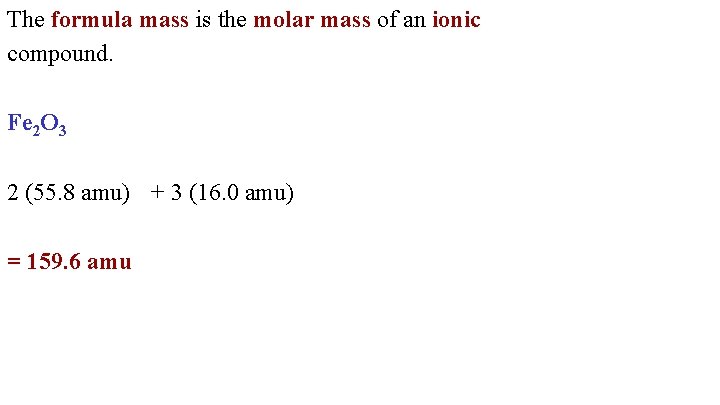
The molecular weight of this compound is 18018gmol.
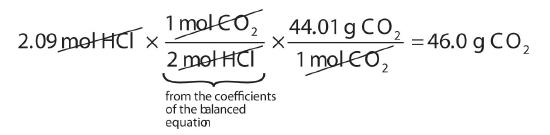
M g X 1 mol X For example consider water with a molar mass of 180 gmol. 02 x 5844 11688 grams. Use the molecular formula to find the molar mass. Calculate the mass of HCl. Molar mass is the mass of a given substance divided by the amount of that substance measured in gmol. The unified atomic mass unit u or dalton Da is a small unit of mass used to express atomic and molecular masses.
 Source: chem.libretexts.org
Source: chem.libretexts.org
In 4788 grams of titanium there is one mole or 6022 x 1023 titanium atoms. Its units are molL moldm 3 or molm 3. ___g H2O x 1 mol H2O 180g H2O ___ mol H2O ___ mol H2O x 180g H2O 1 mol H2O ___ g H2O. Moles mol x Molar Mass gmol 1 x 5844. In order to convert an elements mass.
 Source: slidetodoc.com
Source: slidetodoc.com
When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. N mol m mass in gram M molar. Click to see full answer. Mass g No. 1 mol X m g X.
 Source: pinterest.com
Source: pinterest.com
The molecular weight of this compound is 18018gmol. Grams Moles x Molar Mass. N mol m mass in gram M molar. Type the number of Hydrogen H you want to convert in the text box to see the results in the. Divide the molar mass for the molecular formula by the empirical formula mass.
 Source: youtube.com
Source: youtube.com
Moles mol x Molar Mass gmol 1 x 5844. The molecular weight of this compound is 18018gmol. Molar concentration is the amount of a solute present in one unit of a solution. Divide the molar mass for the molecular formula by the empirical formula mass. Molar Mass of Frequently Calculated Chemicals.
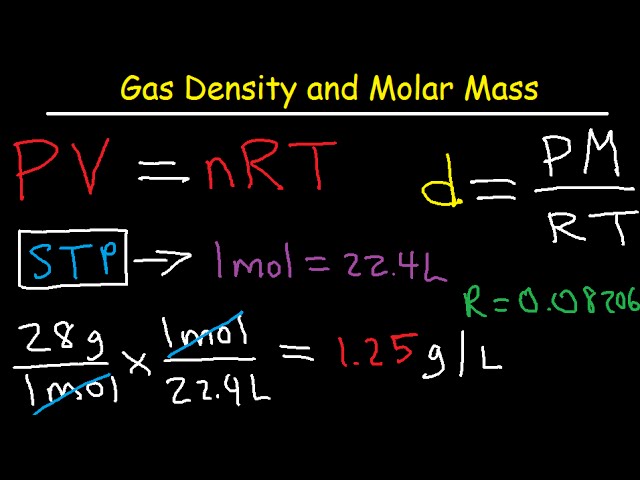 Source: youtube.com
Source: youtube.com
First you must calculate the number of moles in this solution by rearranging the equation. There is a simple relationship between these quantities. Then multiply it by the number of moles to get an answer in grams. Mass g No. The unified atomic mass unit u or dalton Da is a small unit of mass used to express atomic and molecular masses.
 Source: pinterest.com
Source: pinterest.com
Aug 24 2019 Step 2. MOLAR MASS OF AN ELEMENT RELATIVE ATOMIC MASS MOLAR MASS CONSTANT1gmol for HYDROGEN MOLAR MASS of Hydrogen. 1 mol 1 molar mass. The unified atomic mass unit u or dalton Da is a small unit of mass used to express atomic and molecular masses. Mass g No.
 Source: youtube.com
Source: youtube.com
Molar mass is the mass of a given substance divided by the amount of that substance measured in gmol. N mol m mass in gram M molar. For example the atomic mass of titanium is 4788 amu or 4788 gmol. Type the number of Atomic mass unit u you want to convert in the text box to see the results in the table. You just have to know the formula.
 Source: youtube.com
Source: youtube.com
To do this first calculate the molar mass of a sample. Moles mol x Molar Mass gmol 1 x 5844. You just have to know the formula. Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams. Thus for example the average mass of a molecule of water is about 180153 daltons and the molar mass of water is about 180153 gmol.
 Source: pinterest.com
Source: pinterest.com
02 x 5844 11688 grams. ___g H2O x 1 mol H2O 180g H2O ___ mol H2O ___ mol H2O x 180g H2O 1 mol H2O ___ g H2O. Molar Mass of Frequently Calculated Chemicals. 02 x 5844 11688 grams. N mol m mass in gram M molar.
 Source: youtube.com
Source: youtube.com
Might 22 2018 Calculate the molar mass primarily based on the formulation and divide this into the mass of the particular compound. In 4788 grams of titanium there is one mole or 6022 x 1023 titanium atoms. Calculate the mass of 1 L of solution. 1 mol X m g X. MOLAR MASS OF AN ELEMENT RELATIVE ATOMIC MASS MOLAR MASS CONSTANT1gmol for HYDROGEN MOLAR MASS of Hydrogen.
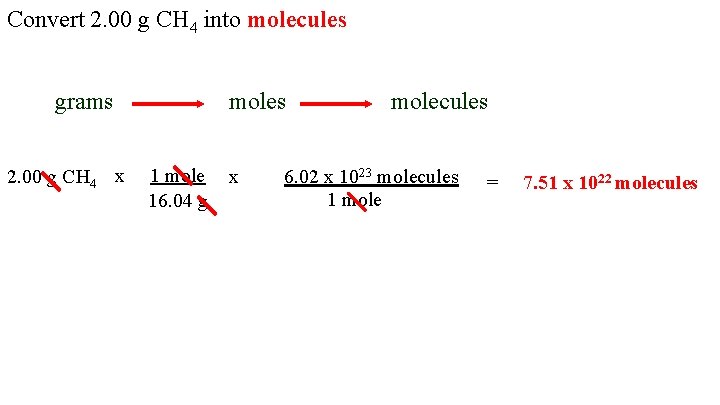 Source: slidetodoc.com
Source: slidetodoc.com
How do you calculate the number of molecules in a mole. The number of grams in the molar mass grams per mole of a molecular compound is the same as its molecular mass. To do this first calculate the molar mass of a sample. The base SI unit for mass is the kilogram and that for amount of substance is the mole. M_s M_s N_s where m_s is the mass of the substance in grams N_s is the quantity of the substance in moles.
 Source: wikihow.com
Source: wikihow.com
Its units are molL moldm 3 or molm 3. 1 mol 1 molar mass. Mass g No. The unified atomic mass unit u or dalton Da is a small unit of mass used to express atomic and molecular masses. Moles mol x Molar Mass gmol 1 x 5844.
 Source: slideplayer.com
Source: slideplayer.com
Convert 02 moles of Sodium chloride. Mass g No. In order to convert an elements mass. By dividing the total mass of the number of moles that you areconsidering by number of moles. Thus the derived unit for molar mass is kgmol.
 Source: pinterest.com
Source: pinterest.com
Molar mass is therefore the mass in grams of 1 mol From which we can construct two conversion factors. Divide the molar mass for the molecular formula by the empirical formula mass. However for both practical and historical reasons molar masses are almost always quoted in grams per mole gmol or g mol 1 especially in chemistry. The base SI unit for mass is the kilogram and that for amount of substance is the mole. First you must calculate the number of moles in this solution by rearranging the equation.
 Source: slideplayer.com
Source: slideplayer.com
The result determines how many times to multiply the subscripts in the empirical formula to get the molecular formula. The number of grams in the molar mass grams per mole of a molecular compound is the same as its molecular mass. 1 mol X m g X. Type the number of Atomic mass unit u you want to convert in the text box to see the results in the table. Might 22 2018 Calculate the molar mass primarily based on the formulation and divide this into the mass of the particular compound.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to turn molar mass into mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






