Your How to switch from moles to grams images are ready. How to switch from moles to grams are a topic that is being searched for and liked by netizens today. You can Get the How to switch from moles to grams files here. Get all free photos.
If you’re looking for how to switch from moles to grams images information connected with to the how to switch from moles to grams topic, you have visit the right site. Our website frequently gives you suggestions for seeking the maximum quality video and image content, please kindly hunt and locate more informative video articles and images that match your interests.
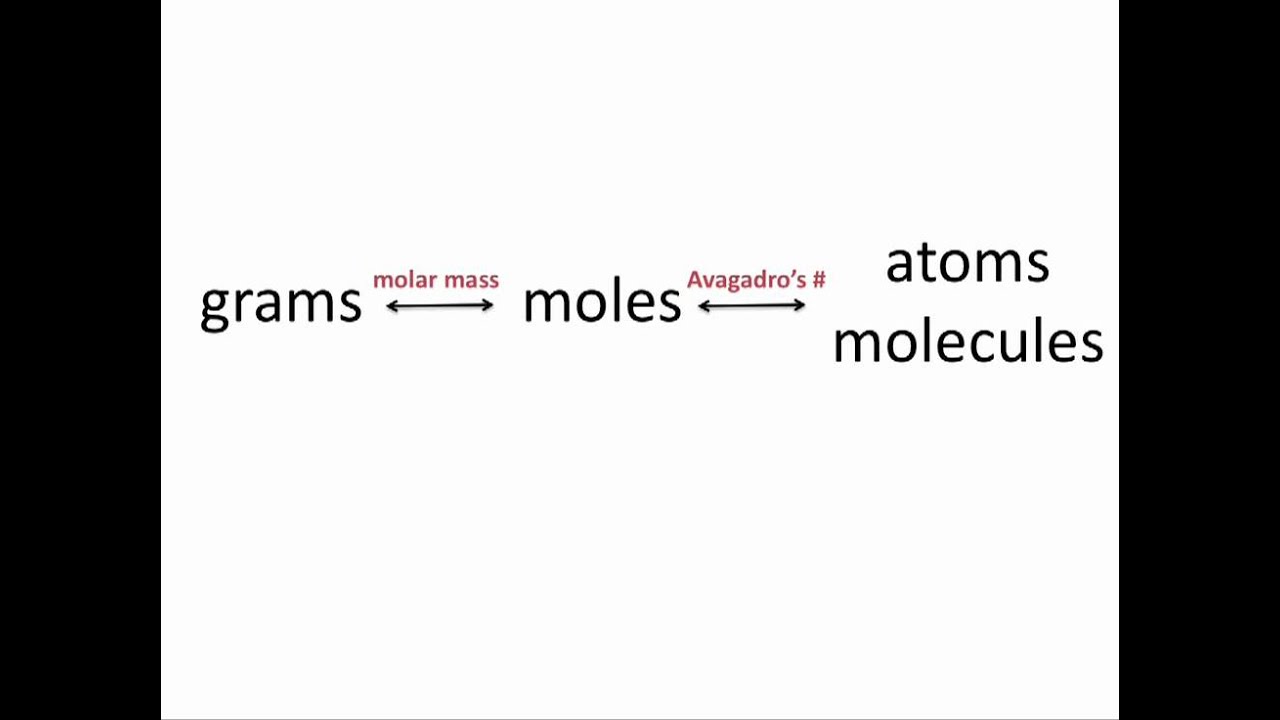
How To Switch From Moles To Grams. Prev Article Next Article. In order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass. The problems start easy and th. You also need to do them using a scientific.
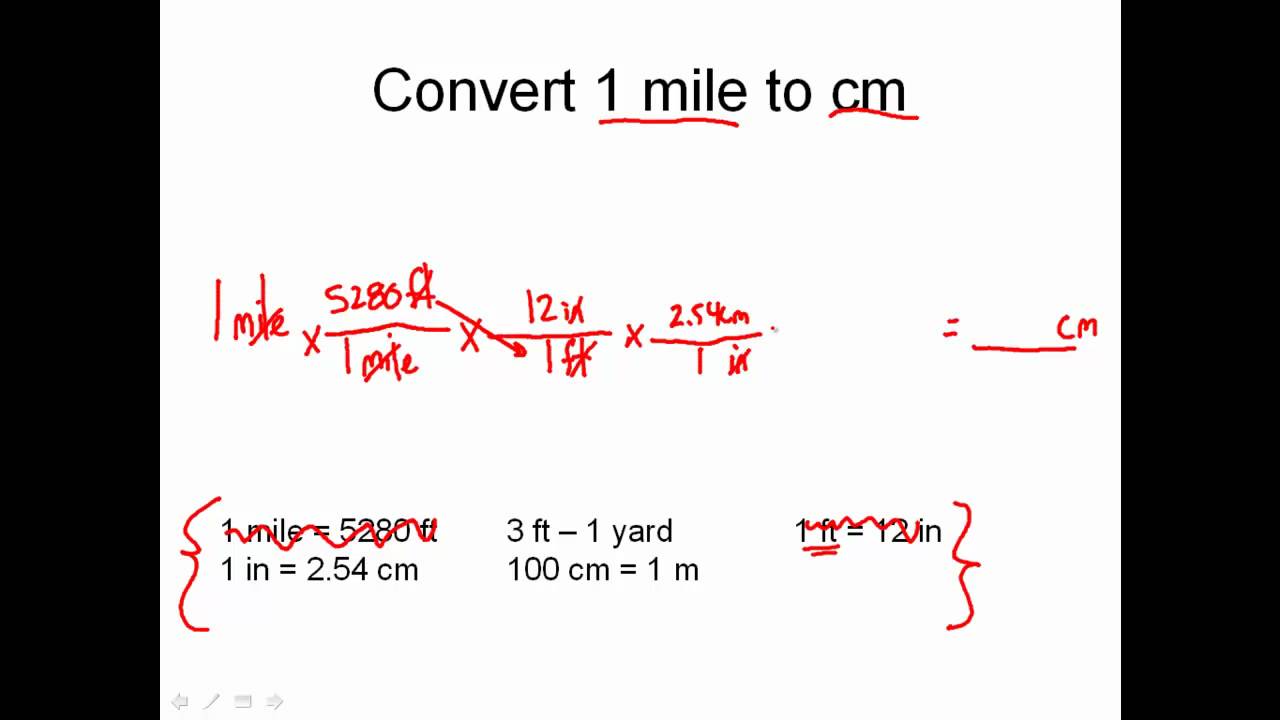 Solving Dimensional Analysis Problems Unit Conversion Problems Made Easy Dimensional Analysis Science Lessons Literal Equations From pinterest.com
Solving Dimensional Analysis Problems Unit Conversion Problems Made Easy Dimensional Analysis Science Lessons Literal Equations From pinterest.com
The simple thing is multiply the molar mass of the substance by moles to get the grams quantity of that particular substance. Look for the atomic masses of hydrogen sulfur and oxygen. What is the detailed process to convert moles to grams. In other words it is the product of the mass of the substance and its molecular weight. Do a quick conversion. To convert the moles into grams multiply the mass of the substance by the molecular weight formula weight.
In other words it is the product of the mass of the substance and its molecular weight.
Litre raised to the power negative 1. Thus the conversion of moles to grams formula is Grams Mass of the substance in moles Molecular weight. Therefore the molecular mass of H. Number of grams of H 2 O. 5 moles to moles 5 moles. Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4.
 Source: pinterest.com
Source: pinterest.com
What is the mass in grams of 0850 moles of sulfur dioxide SO 2. In order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass. I would also like for you to be able to do these kind of calculations using a spreadsheet program like Microsoft Offices Excel or the free one from OpenOffice called Calc. Do a quick conversion. First of all you should check total number of moles given in the problem.
 Source: pinterest.com
Source: pinterest.com
1 moles to moles 1 moles. For water the GFM is 1802 gmol. Converting between moles and grams is a fundamental chemistry skill. Calculate the moles from the grams. Prev Article Next Article.
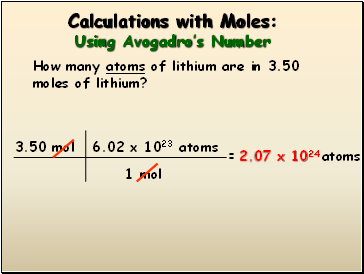 Source: sliderbase.com
Source: sliderbase.com
To convert moles into grams determine the number of moles preset and the molar mass of the compound. This chemistry video tutorial explains how to convert moles to grams which is useful in typical stoichiometry problems. Note that rounding errors may occur so always check the results. Look for the atomic masses of hydrogen sulfur and oxygen. Convert 35 moles of H 2 O to grams.
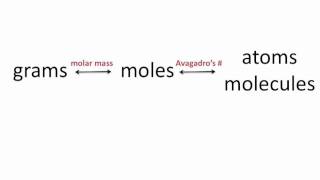 Source: youtube.com
Source: youtube.com
To convert moles into grams determine the number of moles preset and the molar mass of the compound. The formula is Grams Molar Mass of substance Given Moles 4. Write the units based on molar mass information and set up fractions to correctly cancel the units. In other words it is the product of the mass of the substance and its molecular weight. Set up the math in the following format.
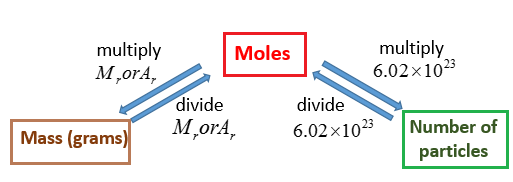 Source: onlinemathlearning.com
Source: onlinemathlearning.com
If starting with grams we use 1 mL193g to convert to milliliters. Suppose you have 16 grams of water. Prev Article Next Article. Therefore for 16 grams of water there is 089 moles. For water the GFM is 1802 gmol.
 Source: youtube.com
Source: youtube.com
Do a quick conversion. This chemistry video tutorial explains how to convert moles to grams which is useful in typical stoichiometry problems. Moles to Grams Step-by-Step Example. 1 mole is equal to 1 moles. 1 moles to moles 1 moles.
 Source: thelablads.blogspot.com
Source: thelablads.blogspot.com
Anything raised to the power -1 is the same. Check the chart for more details. The formula for moles to grams is given by. Since water has two molecules of hydrogen and one molecule of oxygen then the molecular weight of water is 1801528gmol. More commonly written for this application as.
 Source: pinterest.com
Source: pinterest.com
Suppose you have 16 grams of water. Litre raised to the power negative 1. To convert the moles into grams multiply the mass of the substance by the molecular weight formula weight. 1 mole is equal to 1 moles. Write the units based on molar mass information and set up fractions to correctly cancel the units.
 Source: khanacademy.org
Source: khanacademy.org
Calculate the moles from the grams. The formula is Grams Molar Mass of substance Given Moles 4. Litre raised to the power negative 1. In other words it is the product of the mass of the substance and its molecular weight. The simple thing is multiply the molar mass of the substance by moles to get the grams quantity of that particular substance.
 Source: pinterest.com
Source: pinterest.com
Prev Article Next Article. Convert 139 mol of water to grams of water. Prev Article Next Article. What is the detailed process to convert moles to grams. The problems start easy and th.
 Source: youtube.com
Source: youtube.com
Determine the number of moles The number of moles present in a compound is often given to the student in the problem. The problems start easy and th. What is the detailed process to convert moles to grams. Multiply the moles given by the substances molar mass. Moles to Grams Conversion Formula On mole is made up of total number of Avogadro atoms and If you are sure of quantity of moles then they are converted to grams quickly.
 Source: wikihow.com
Source: wikihow.com
Here we use the other conversion factor. Here we use the other conversion factor. What is the detailed process to convert moles to grams. As you already know how the grams to moles conversion work find the number of moles. Anything raised to the power -1 is the same.
 Source: pinterest.com
Source: pinterest.com
Moles to Grams Conversion Formula On mole is made up of total number of Avogadro atoms and If you are sure of quantity of moles then they are converted to grams quickly. Thus the conversion of moles to grams formula is Grams Mass of the substance in moles Molecular weight. N 5988 g 18015 gmol 3324 mol. What is the mass in grams of 0850 moles of sulfur dioxide SO 2. How many moles are in 327 grams of.
 Source: youtube.com
Source: youtube.com
Type in your own numbers in the form to convert the units. This chemistry video tutorial explains how to convert moles to grams which is useful in typical stoichiometry problems. The mass of the substance must be in grams. The problems start easy and th. Multiply moles by the molar mass to convert from moles to grams and divide grams by the molar mass to convert from grams to moles.
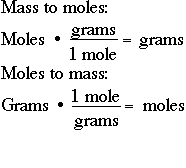 Source: socratic.org
Source: socratic.org
Now calculate the molar mass of the substance. Answer 1 of 11. The problems start easy and th. Once you have both the molecular mass and physical weight of the compound you can use a simple formula to calculate the number of moles present in the sample. Use this page to learn how to convert between moles and moles.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to switch from moles to grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






