Your How to prepare 1 mole of hcl images are available. How to prepare 1 mole of hcl are a topic that is being searched for and liked by netizens today. You can Download the How to prepare 1 mole of hcl files here. Find and Download all royalty-free vectors.
If you’re searching for how to prepare 1 mole of hcl images information related to the how to prepare 1 mole of hcl topic, you have come to the right site. Our website frequently gives you suggestions for seeing the maximum quality video and image content, please kindly search and find more informative video content and images that match your interests.
How To Prepare 1 Mole Of Hcl. 0 1 a. How much concentrated solution would you take to prepare 280L of 0475M HCl by mixing with water. Concentrated Hydrochloric Acid Solution A 1 mole HCl in 1000 mL weigh 365g RMM of HCL 1 355 However Sol. Subsequently question is what is M HCl.
 How To Prepare 0 1 Molar Hcl Solution Youtube From youtube.com
How To Prepare 0 1 Molar Hcl Solution Youtube From youtube.com
Molarity Normalityn-factor02102 M 02 moles should be present in 1 litre of solution. Volume and weight of the substance is calculated using its molecular weight density and number of moles. 1 gram equivalent of HCl 1 mole of HCl. Let us assume that we want to prepare 1 L of 1 molL HCl. 5 lo g a 0. Calculate the number of moles of HCl.
Calculate the moles of HCl needed.
Volume and weight of the substance is calculated using its molecular weight density and number of moles. Calculate the molarity of an HCl solution which contains 1823 g ofHCl in 3550 mL of solution. 90 M HCl 90 moles of HCl per liter of solution. 12M 37 HCL 12 molesL 12 x 365 438 gL 438 mgml. Molarity moles of solute volume of solution in liters If you know two of these quantities you can find. One mole of hydrochloric acid 365 g.
 Source: youtube.com
Source: youtube.com
A its Specific Gravity gmL x 1000 116 x 1000 1160g. You can see 01 mol dm-3 HCl solution is strong acidic solution because pH value is well below seven. The molar mass of hydrochloric acid is calculated as under. 12M 37 HCL 12 molesL 12 x 365 438 gL 438 mgml. To make 1 L of 1 molL HCl we take 88 mL of the concentrated solution and add water to make a total of 1 L.
 Source: youtube.com
Source: youtube.com
We have to dissolve 241433 g of AlCl36H2O 96 purity in deionized or distilled water. Normality Molarity x n-factor. One mole of hydrochloric acid 365 g. Do watch and let me know your problems in chemistry. Let us assume that we want to prepare 1 L of 1 molL HCl.
 Source: slideplayer.com
Source: slideplayer.com
Chapter 5 Molarity can be used as a conversion factor. Therefore taking 493- ml of. PH -log 10 01 pH 1. The difference between 1 molar of HCl and 2 molar of HCl is that the 2 molar HCl is more concentrated. Enter number of moles and select a substance to search for.
 Source: toppr.com
Source: toppr.com
0 1 a lo g 4. SolCalc - Laboratory Report September 03 2012 132020 HYDROCHLORIC ACID cHCl 01 molL To prepare 500 mL of a 01 molL solution of hydrochloric acid we will need to dilute 422917 mL of 365 HCl to a final volume of. How much concentrated solution would you take to prepare 280L of 0475M HCl by mixing with water. Convert moles to volume and weight. So 05 gram equivalent HCl 05 mole HCl 05 x 365 gram HCl 1825 g HCl.
 Source: youtube.com
Source: youtube.com
37 HCl means 37- g of HCl is present in 100- ml of the solution. Calculate the mass of HCl Needed. Moles of HCl1L HCl times frac1 mol HCl1L HCl Moles of HCl 1 mol HCl. Convert moles to volume and weight. 0 1 a lo g 4.
 Source: youtube.com
Source: youtube.com
H C N a Applying the equation p H lo g H C N N a C N lo g K a 8. Calculate the mass of HCl Needed. Calculate the moles of HCl needed. H C N a Applying the equation p H lo g H C N N a C N lo g K a 8. 1 2 9 6 0.
 Source: toppr.com
Source: toppr.com
02moles 02 x 365 73 grams of HCl Dissolve 73 grams. 0 1 0. It means that in 1 liter of HCl solution 1 mole of HCl will be present. Molarity Normalityn-factor02102 M 02 moles should be present in 1 litre of solution. Calculate the number of moles of HCl.
 Source: youtube.com
Source: youtube.com
Use as a wildcard for partial matches or enclose the search string in double quotes for an exact match. Click hereto get an answer to your question How many mole of HCl will be required to prepare one litre of buffer solution containing NaCN HCI of pH 85 using 001 g formula weight of NaCN Ka HCN 4 x 10-10 AL 01 126 A 685 x 10-3 mol 885 x 10-3 mol 765 x 10-3 mol 0 485 x 103 mol. Calculate the mass of HCl Needed. 0 1 0. This compound is also known as Hydrochloric Acid.
 Source: youtube.com
Source: youtube.com
5 lo g a 0. This compound is also known as Hydrochloric Acid. 0 1 a lo g 4. H concentration is very high in those solution. 12M 37 HCL 12 molesL 12 x 365 438 gL 438 mgml.
 Source: youtube.com
Source: youtube.com
Calculate the moles of HCl needed. To prepare 1000 mL of a 01 molL solution of Hydrochloric acid. 1 gram equivalent of HCl 1 mole of HCl. Cm 31000 cm 3 dm 33646 g HClmol HCl 120 M approximate. PH of laboratory HCl bottles.
 Source: youtube.com
Source: youtube.com
One mole of hydrochloric acid 365 g. The indicated density is compatible with this value. 1 grams HCl 0027426610504282 mole using the molecular weight calculator and the molar mass of HCl. The definition of molarity contains 3 quantities. 02moles 02 x 365 73 grams of HCl Dissolve 73 grams.
 Source: m.youtube.com
Source: m.youtube.com
1 grams HCl 0027426610504282 mole using the molecular weight calculator and the molar mass of HCl. Calculate the molarity of an HCl solution which contains 1823 g ofHCl in 3550 mL of solution. H concentration is very high in those solution. Calculate the number of moles of HCl. Chapter 5 Molarity can be used as a conversion factor.
 Source: youtube.com
Source: youtube.com
12M 37 HCL 12 molesL 12 x 365 438 gL 438 mgml. Mass of HCl1 mol HCl times frac3646 g of HCl1 mol of HCl Mass of HCl 3646 g HCl. This compound is also known as Hydrochloric Acid. The indicated density is compatible with this value. Molar mass of HCl 3646094 gmol.
 Source: youtube.com
Source: youtube.com
Therefore taking 493- ml of. 1 gram equivalent of HCl 1 mole of HCl. So 1M HCl would be 365 g of hydrochloric acid dissolved in 1 litre of solvent. Hydrochloric acid is usually purchased in a concentrated form that is 370 HCl by mass and has a density of 120gmL. 1823 g HCl 1 mol HCl 3646 g HCl 05000mol HCl.
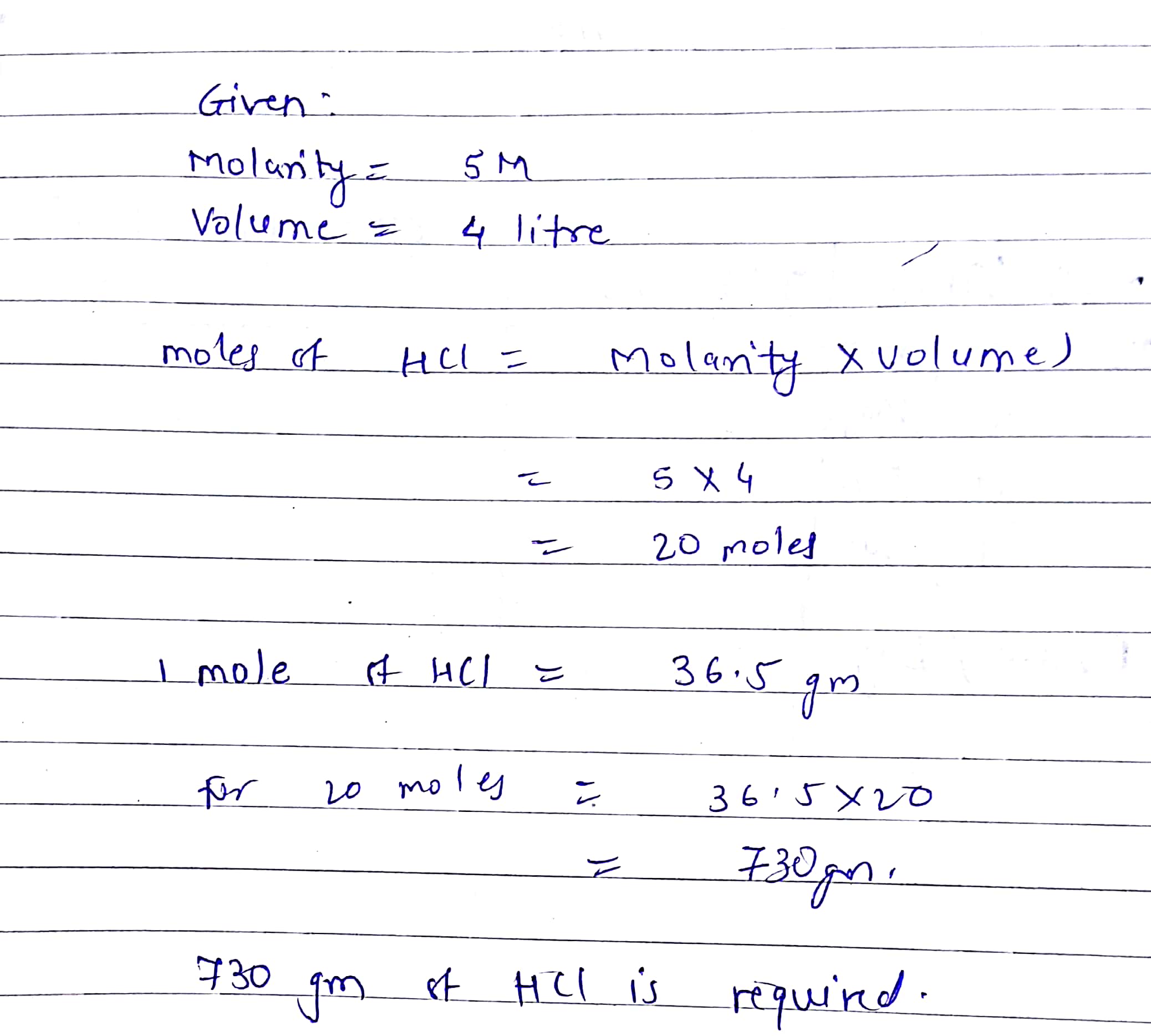 Source: topperlearning.com
Source: topperlearning.com
PH -log 10 HCl aq pH of 01 mol dm-3 HCl solution. Calculate the mass of HCl Needed. The definition of molarity contains 3 quantities. So 05 gram equivalent HCl 05 mole HCl 05 x 365 gram HCl 1825 g HCl. Use as a wildcard for partial matches or enclose the search string in double quotes for an exact match.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to prepare 1 mole of hcl by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






