Your How to go to moles to grams images are available. How to go to moles to grams are a topic that is being searched for and liked by netizens now. You can Download the How to go to moles to grams files here. Download all free photos and vectors.
If you’re searching for how to go to moles to grams images information connected with to the how to go to moles to grams keyword, you have come to the right site. Our website frequently gives you hints for downloading the maximum quality video and image content, please kindly hunt and locate more informative video articles and images that fit your interests.
How To Go To Moles To Grams. N m M where M is the molar mass of this material. The amount that one mole of fuel occupies at STP 224Lmol Mole to Mole Ratio. If it is not then it can be found by looking at the. As a unit of measurement the mole is like a dozen or a grossA dozen eggs means twelve eggs whereas a gross of pencils.
 Chemistry Lessons Chemistry Classroom Teaching Chemistry From pinterest.com
Chemistry Lessons Chemistry Classroom Teaching Chemistry From pinterest.com
Divide the number of grams of the compound NaOH by the molecular weight and as a result the grams g unit cancels and all we have left is. Calculate the time utilizing the present and the coulombs of cost. The amount that one mole of fuel occupies at STP 224Lmol Mole to Mole Ratio. Your goal is to determine the mass in grams of two moles of carbon. 0700 mole x 340146 gramsmole 238 grams The answer of 238 g has been rounded to three significant figures because the 0700 value had the least number of significant figures in the problem. You want a formulation and a periodic desk.
7 moles in to grams 803726 gramsBased on that information to convert 1009 moles of curium to grams we multiply 1009 moles of curium by 247.
Moles To Grams Formula. Divide the number of grams of the compound NaOH by the molecular weight and as a result the grams g unit cancels and all we have left is. N m M where M is the molar mass of this material. TextMass In Grams textNo. 0700 mole x 340146 gramsmole 238 grams The answer of 238 g has been rounded to three significant figures because the 0700 value had the least number of significant figures in the problem. How to go from moles to grams.
 Source: pinterest.com
Source: pinterest.com
This dimensional analysis video tuto. M n M The same formula is used by the free online moles to grams calculator to generate accurate results. The mole is the SI unit of the measurement for the amount of a substance. Where is the molar mass of the substance. N m M the place.
 Source: pinterest.com
Source: pinterest.com
In order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass. N m M where M is the molar mass of this material. To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. Multiply the number of moles by the molar mass to obtain the final answer in grams. You want a formulation and a periodic desk.
 Source: pinterest.com
Source: pinterest.com
A video made by a student for a student. Multiply the moles given by the substances molar mass. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. 7 moles in to grams 803726 gramsBased on that information to convert 1009 moles of curium to grams we multiply 1009 moles of curium by 247. As a unit of measurement the mole is like a dozen or a grossA dozen eggs means twelve eggs whereas a gross of pencils.
 Source: pinterest.com
Source: pinterest.com
If it is not then it can be found by looking at the. The unit is typically gmol. 139 mol H₂O 1802 g H2O 1 mol H2O 250 g H₂O. Moles to Grams Conversion Formula Questions. Calculate the time utilizing the present and the coulombs of cost.
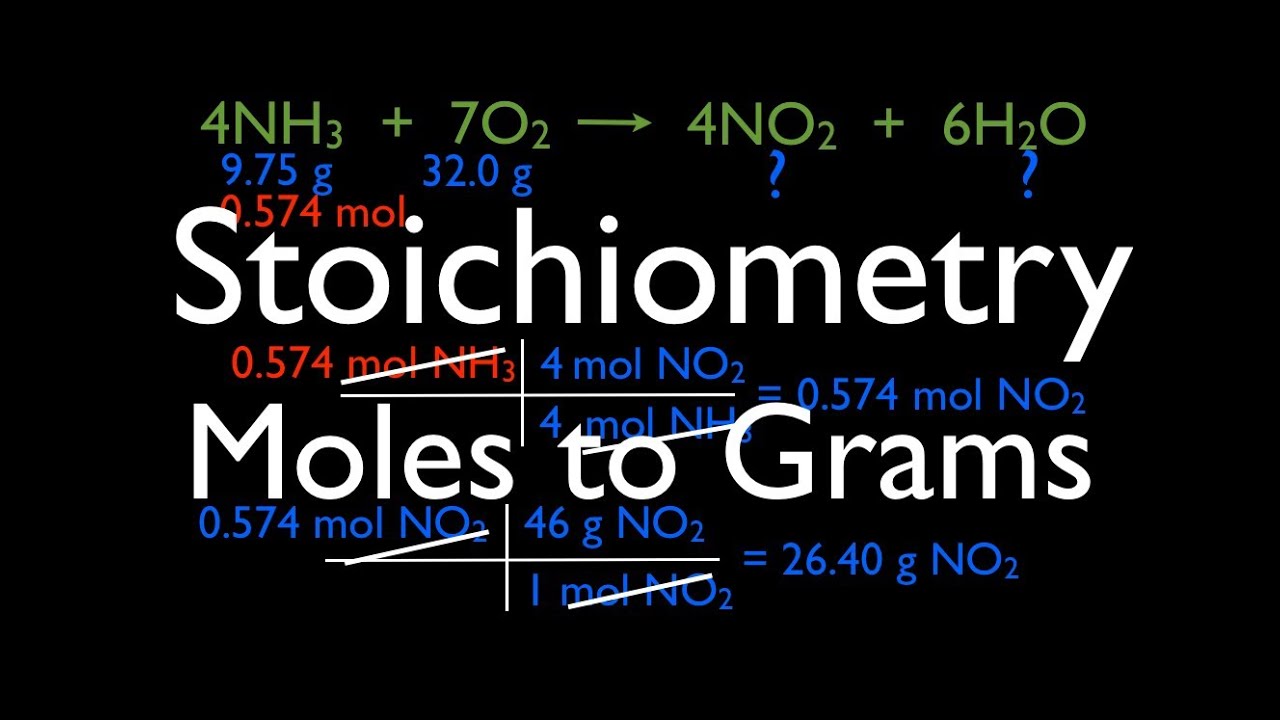 Source: pinterest.com
Source: pinterest.com
Here we use the other conversion factor. Multiply the number of moles by the molar mass to obtain the final answer in grams. In order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass. Here we use the other conversion factor. To convert from molecules to grams it is necessary to first convert the number of molecules of a substance by dividing by Avogadros number to find the number of moles and then multiply the number of moles by the molar mass of this substance.
 Source: co.pinterest.com
Source: co.pinterest.com
But wait what actually is a mole. M n M The same formula is used by the free online moles to grams calculator to generate accurate results. Determine the number of moles. Each jar holds exactly one mole of carbon. This dimensional analysis video tuto.
 Source: pinterest.com
Source: pinterest.com
Moles To Grams Formula. 250 g H₂O 1 mol H2O 1802 g H2O 139 mol H₂O. 7 moles in to grams 803726 gramsBased on that information to convert 1009 moles of curium to grams we multiply 1009 moles of curium by 247. This chemistry video tutorial explains how to convert moles to grams which is useful in typical stoichiometry problems. Moles To Grams Formula.
 Source: pinterest.com
Source: pinterest.com
Divide the number of grams of the compound NaOH by the molecular weight and as a result the grams g unit cancels and all we have left is. 139 mol H₂O 1802 g H2O 1 mol H2O 250 g H₂O. N m M the place. The formula for moles to grams is given by Grams Moles times AtomicWeight. This chemistry video tutorial explains how to convert moles to grams which is useful in typical stoichiometry problems.
 Source: pinterest.com
Source: pinterest.com
Moles to Grams Conversion Formula Questions. You can carry out moles to grams conversion with the help of the following equation below. 0700 mole x 340146 gramsmole 238 grams the answer of 238 g has been rounded to three significant figures because the 0700 value had the least number of significant figures. To convert from molecules to grams it is necessary to first convert the number of molecules of a substance by dividing by Avogadros number to find the number of moles and then multiply the number of moles by the molar mass of this substance. Your goal is to determine the mass in grams of two moles of carbon.
 Source: pinterest.com
Source: pinterest.com
But wait what actually is a mole. This chemistry video tutorial explains the conversion process of atoms to grams which is a typical step in common dimensional analysis stoichiometry problems. Set the amount to 2000 moles of carbon mol C then press Start. Moles to Grams Conversion Formula Questions. Moles To Grams Formula.
 Source: pinterest.com
Source: pinterest.com
How do you convert particles to grams and grams to particles. You want a formulation and a periodic desk. Moles to Grams Conversion Formula Questions. Relating to this how is a mole ratio from a response utilized in stoichiometric issues. If it is not then it can be found by looking at the.
 Source: pinterest.com
Source: pinterest.com
Divide the number of grams of the compound NaOH by the molecular weight and as a result the grams g unit cancels and all we have left is. Where is the molar mass of the substance. Moles To Grams Formula. 7 moles in to grams 803726 gramsBased on that information to convert 1009 moles of curium to grams we multiply 1009 moles of curium by 247. Multiply the number of moles by the molar mass to obtain the final answer in grams.
 Source: pinterest.com
Source: pinterest.com
To convert from molecules to grams it is necessary to first convert the number of molecules of a substance by dividing by Avogadros number to find the number of moles and then multiply the number of moles by the molar mass of this substance. Convert 139 mol of water to grams of water. N m M where M is the molar mass of this material. If it is not then it can be found by looking at the. Relating to this how is a mole ratio from a response utilized in stoichiometric issues.
 Source: pinterest.com
Source: pinterest.com
Youll need to know the volume of water used. Moles to Grams Conversion Formula Questions. Determine the number of moles. N m M where M is the molar mass of this material. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula.
 Source: hu.pinterest.com
Source: hu.pinterest.com
Multiply the moles given by the substances molar mass. Here we use the other conversion factor. 0700 mole x 340146 gramsmole 238 grams the answer of 238 g has been rounded to three significant figures because the 0700 value had the least number of significant figures. Divide the number of grams of the compound NaOH by the molecular weight and as a result the grams g unit cancels and all we have left is. In order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to go to moles to grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






