Your How to go from moles to grams in stoichiometry images are available. How to go from moles to grams in stoichiometry are a topic that is being searched for and liked by netizens today. You can Find and Download the How to go from moles to grams in stoichiometry files here. Find and Download all free images.
If you’re looking for how to go from moles to grams in stoichiometry images information connected with to the how to go from moles to grams in stoichiometry interest, you have visit the ideal site. Our site always gives you hints for downloading the highest quality video and image content, please kindly surf and locate more enlightening video articles and images that fit your interests.
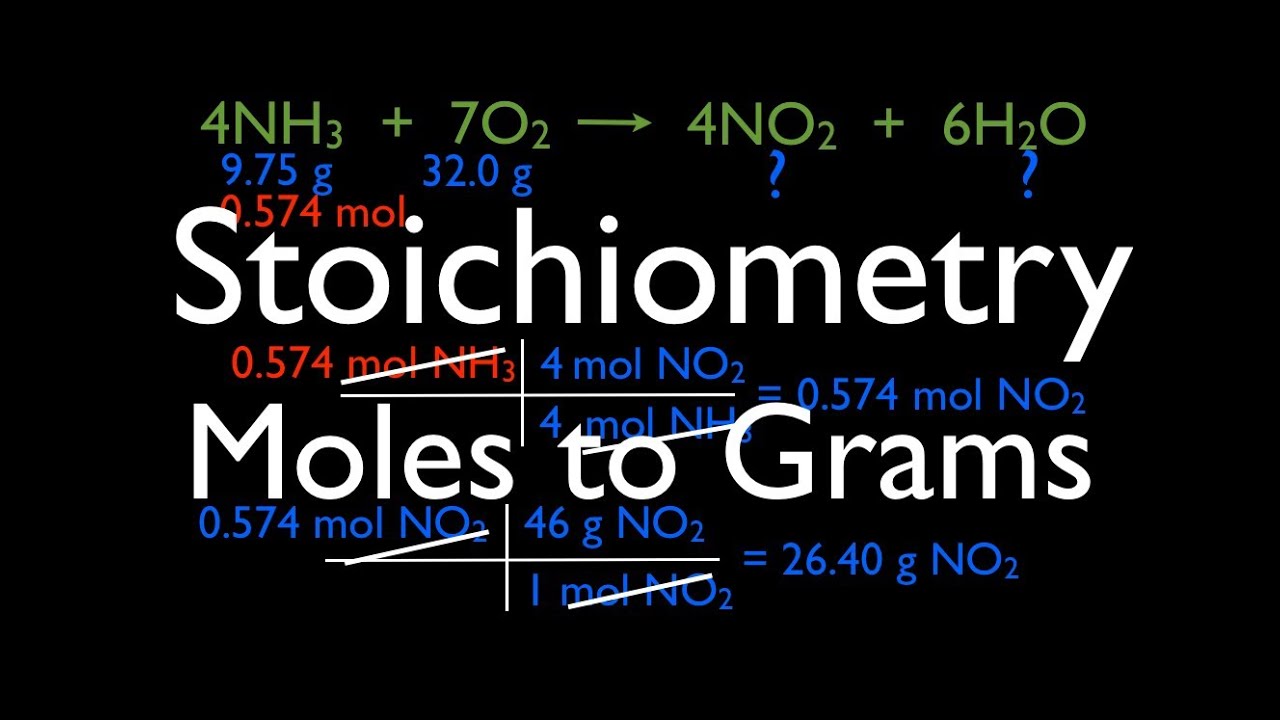
How To Go From Moles To Grams In Stoichiometry. Here we know that 1 mole of. The stoichiometric ratio that will be used is 1 mole Al2SO43 12 moles O 1 m o l. Stoichiometry practice problems worksheet answers pdf. Go on the bottom to cancel the mol CaOH 2 on top.
 Stoichiometry Grams To Moles Teaching Chemistry Chemistry Classroom Science Chemistry From pinterest.com
Stoichiometry Grams To Moles Teaching Chemistry Chemistry Classroom Science Chemistry From pinterest.com
How do you determine the gram formula mass. Write and balance the chemical equation. Example 1 0200 mole of H 2 O contains how many molecules. You will use molar mass again but this time you will multiply to convert moles back to grams. Convert the following number of moles of chemical into its corresponding mass in grams. 2K S K2S The coefficient ratio between K S is 21.
Example 4 When the word gram replaces mole you have a related set of problems which requires one more step.
2 agno 3 bacl 2. Coefficients give mole ratios. Go on the bottom to cancel the mol CaOH 2 on top. 2 naoh h 2so 4 2 h 2o na 2so 4 how many grams of sodium sulfate will be formed if you start with 200 0. First we have to calculate the number of moles of oxygen present in the compound. Example 4 When the word gram replaces mole you have a related set of problems which requires one more step.
 Source: pinterest.com
Source: pinterest.com
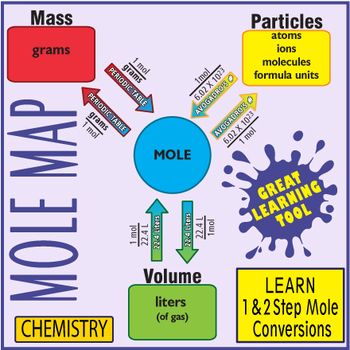
Then the moles of the unknown are converted into mass in grams by use of the molar mass of that substance from the periodic table. Coefficients give mole ratios. Use the conversion factor. - Molarity molL is a relation between moles of a solute and volume of the solution and is a useful conversion factor in stoichiometry. 0700 mole x 340146 gramsmole 238 grams the answer of 238 g has been rounded to three significant figures because the 0700 value had the least number of significant figures.
 Source: gr.pinterest.com
Source: gr.pinterest.com
Practice converting moles to grams and from grams to moles when given the molecular weight. Example 4 When the word gram replaces mole you have a related set of problems which requires one more step. This chemistry video tutorial explains how to convert moles to grams which is useful in typical stoichiometry problems. Example 1 0200 mole of H 2 O contains how many molecules. 0450 mole of Fe contains how many atoms.
 Source: pinterest.com
Source: pinterest.com
2 atoms of K react with 1 atom of S. The stoichiometric ratio that will be used is 1 mole Al2SO43 12 moles O 1 m o l. Add up 1 Hydrogen and 1 Chlorine. 962 grams H 2 O x 1 mole H 2 O1802 grams 962 x 1 mole 1802 053 moles Check to see if your answer makes sense. You are given the moles of one component and needed to find the volume of another gaseous component.
 Source: pinterest.com
Source: pinterest.com
O We use this mole ratio to convert from moles of B 2 H 6 to moles of O 2. Method 1 watch video tutorial. - Stoichiometry calculations are used to convert grams moles and volume using molarity by relating amounts of reactants and products in a balanced chemical equation. Practice converting moles to grams and from grams to moles when given the molecular weight. 392 mol O 2 32 g O 2 1 mol O 2 1253g O 2.
 Source: pinterest.com
Source: pinterest.com
Put the mols on the bottom so that it cancels leaving grams on top. 2 naoh h 2so 4 2 h 2o na 2so 4 how many grams of sodium sulfate will be formed if you start with 200 0. This chemistry video tutorial provides a basic introduction into stoichiometry. Use the conversion factor. Stoichiometry practice problems worksheet answers pdf.
 Source: pinterest.com
Source: pinterest.com
Then the moles of the unknown are converted into mass in grams by use of the molar mass of that substance from the periodic table. Example 3 0200 gram of H 2 O contains how many molecules. 447 g CaOH 2 1 mol CaOH 2 2 mol HCl 74094 g CaOH 21 mol CaOH 2 The last step is to change the mols HCl back to grams. Also means that 2 moles of K react with 1 mole of S. When going from liters to moles you divide.
 Source: pinterest.com
Source: pinterest.com
Convert moles back to mass using the molar mass of the species. 1 mol gas 22. You need a formula and a periodic table. This chemistry video tutorial explains how to convert moles to grams which is useful in typical stoichiometry problems. Practice converting moles to grams and from grams to moles when given the molecular weight.
 Source: pinterest.com
Source: pinterest.com
Coefficients give mole ratios. O We use this mole ratio to convert from moles of B 2 H 6 to moles of O 2. Calculate the moles of e-required to supply the moles of Zn utilizing the stoichiometry of the balanced half-reaction. 2 naoh h 2so 4 2 h 2o na 2so 4 how many grams of sodium sulfate will be formed if you start with 200 0. This chemistry video tutorial provides a basic introduction into stoichiometry.
 Source: pinterest.com
Source: pinterest.com
If youre seeing this message it means were having trouble loading external resources on our website. Convert 56 grams N 2 to moles of N 2. How do you determine the gram formula mass. Coefficients give mole ratios. Example 4 When the word gram replaces mole you have a related set of problems which requires one more step.
 Source: pinterest.com
Source: pinterest.com
Write and balance the chemical equation. Watch video tutorial When going from moles to liters you multiply by 224. It contains mole to mole conversions grams to grams and mole to gram dimens. Add up 1 Hydrogen and 1 Chlorine. When going from liters to moles you divide.
 Source: pinterest.com
Source: pinterest.com
Add up 1 Hydrogen and 1 Chlorine. This chemistry video tutorial explains how to convert moles to grams which is useful in typical stoichiometry problems. The moles of the given substance are first converted into moles of the unknown by using the mole ratio from the balanced chemical equation. 1 mol gas 22. - Stoichiometry calculations are used to convert grams moles and volume using molarity by relating amounts of reactants and products in a balanced chemical equation.
 Source: pinterest.com
Source: pinterest.com
962 grams H 2 O x 1 mole H 2 O1802 grams 962 x 1 mole 1802 053 moles Check to see if your answer makes sense. Add up 1 Hydrogen and 1 Chlorine. 2K S K2S The coefficient ratio between K S is 21. Mass of one mole of given substance expressed in gmol The molar mass of a substance in grams has the same numerical value as its relative atomic molecular weight Molar Volume one mole of any gaseous substance occupies the same volume at the same temperature and pressure22414 litres at 101325 kPa 0 C 27315 K Avogadro. It contains mole to mole conversions grams to grams and mole to gram dimens.
 Source: pinterest.com
Source: pinterest.com
How to go from moles to grams. 0450 mole of Fe contains how many atoms. 2 naoh h 2so 4 2 h 2o na 2so 4 how many grams of sodium sulfate will be formed if you start with 200 0. 0700 mole x 340146 gramsmole 238 grams the answer of 238 g has been rounded to three significant figures because the 0700 value had the least number of significant figures. Watch video tutorial When going from moles to liters you multiply by 224.
 Source: pinterest.com
Source: pinterest.com
How to go from moles to grams. Convert the following number of moles of chemical into its corresponding mass in grams. Practice problems chapter 5. - Stoichiometry calculations are used to convert grams moles and volume using molarity by relating amounts of reactants and products in a balanced chemical equation. Mass of one mole of given substance expressed in gmol The molar mass of a substance in grams has the same numerical value as its relative atomic molecular weight Molar Volume one mole of any gaseous substance occupies the same volume at the same temperature and pressure22414 litres at 101325 kPa 0 C 27315 K Avogadro.
 Source: pinterest.com
Source: pinterest.com
Also means that 2 moles of K react with 1 mole of S. 392 mol O 2 32 g O 2 1 mol O 2 1253g O 2. Put the mols on the bottom so that it cancels leaving grams on top. The stoichiometric ratio that will be used is 1 mole Al2SO43 12 moles O 1 m o l. Example 3 0200 gram of H 2 O contains how many molecules.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to go from moles to grams in stoichiometry by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






