Your How to go from moles to atoms images are available in this site. How to go from moles to atoms are a topic that is being searched for and liked by netizens now. You can Find and Download the How to go from moles to atoms files here. Get all royalty-free photos and vectors.
If you’re looking for how to go from moles to atoms images information related to the how to go from moles to atoms topic, you have come to the right site. Our website frequently gives you suggestions for seeing the maximum quality video and image content, please kindly hunt and find more enlightening video articles and graphics that fit your interests.
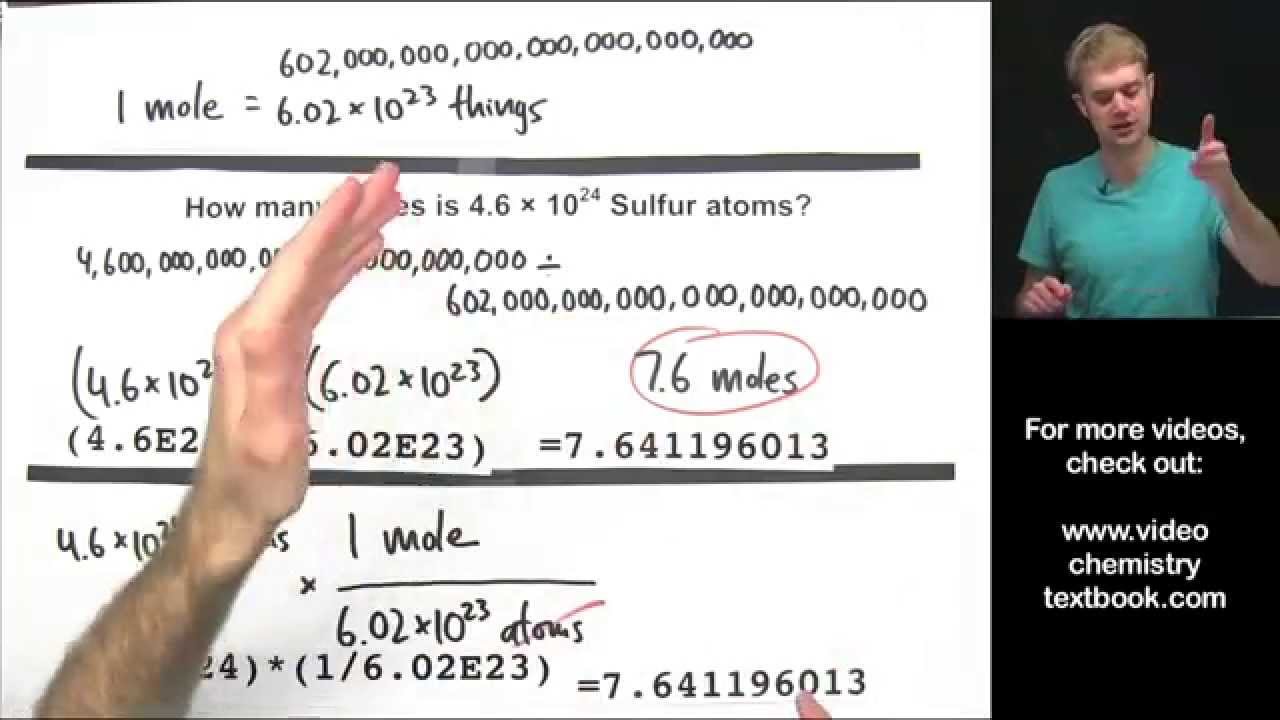
How To Go From Moles To Atoms. The simple unit conversion tool which helps you to convert atoms to moles or moles to atoms. As a simple example a molecule of water H2O is made out of one hydrogen atom and two oxygen atoms. Where Atom Number of atoms. To convert from moles to atoms multiply the molar amount by Avogadros number.
 How To Convert Grams Moles Atoms And Liters In Chemistry In 2021 School Organization Notes School Study Tips Chemistry Quotes From pinterest.com
How To Convert Grams Moles Atoms And Liters In Chemistry In 2021 School Organization Notes School Study Tips Chemistry Quotes From pinterest.com
Quick conversion chart of mole to atom. 301 x 1023 atoms Cu. Since water has a chemical formula of H2O there will be 2 moles of hydrogen in every mole of water. Then you multiply that by your 878 grams. 1 mole 60221023 6022 10 23 atoms molecules protons etc. 12g CO 2 X 2 atoms.
In one mole of water there will exist approximately 6021023 water molecules.
3 mole to atom 180664245E24 atom. People found this article helpful. Mole Conversion Circulation Chart Graphic Chemistry Mole Binder Chemistry Classes Mole Conversion Finding out Targets This chemistry video tutorial explains the conversion technique of atoms. Quick conversion chart of mole to atom. After you get that answer you can use Avagadros number 6022X1023 to find the atoms. Simply put one mole of a substance atoms molecules ions etc.
 Source: pinterest.com
Source: pinterest.com
To convert from atoms to moles divide the atom amount by Avogadros number or multiply by its reciprocal. The Avogadros Constant is equal to 6. 1 mole is equal to 60221415E23 atom. Kilomoles to Moles. 1 mole latex6022times1023latexatoms molecules protons etc.
 Source: pinterest.com
Source: pinterest.com
You divide and find that 1 gram of fluorine is equal to 00525350025878 moles. At STP 1 mole or 602 x 1023 representative particles of any gas occupies a volume of 224 L. 5g carbon 0417 mols of carbon 12 gmol How many moles of oxygen are in 12 g of CO 2. After you get that answer you can use Avagadros number 6022X1023 to find the atoms. Quick conversion chart of mole to atoms.
 Source: pinterest.com
Source: pinterest.com
Avogadros number is a very important relationship to remember. Since water has a chemical formula of H2O there will be 2 moles of hydrogen in every mole of water. Then you multiply that by your 878 grams. 6022 10 23 atoms 1 mol or 1 mol 6022 1023 atoms Converting between Number of Moles and Number of Atoms. 2 mole to atoms 12044283E24 atoms.
 Source: pinterest.com
Source: pinterest.com
To get moles from atoms divide number of atoms by 6022 x 1023. To get atoms from moles multiply number of moles by 6022 x 1023. Use this page to learn how to convert between moles and atoms. 5 mole to atom 301107075E24 atom. Kilomoles to Moles.
 Source: pinterest.com
Source: pinterest.com
1 mole 60221023 6022 10 23 atoms molecules protons etc. Type in your own numbers in the form to convert the units. 1 mole to atoms 60221415E23 atoms. People found this article helpful. You divide and find that 1 gram of fluorine is equal to 00525350025878 moles.
 Source: co.pinterest.com
Source: co.pinterest.com
Where Atom Number of atoms. 1 mole to atom 60221415E23 atom. After you get that answer you can use Avagadros number 6022X1023 to find the atoms. Moles to Atoms Write this. The equation to convert moles to atoms is as follows.
 Source: pinterest.com
Source: pinterest.com
Type in your own numbers in the form to convert the units. Avogadros number is a very important relationship to remember. This chemistry video tutorial explains the conversion process of atoms to grams which is a typical step in common dimensional analysis stoichiometry problems. So there will be a total of 60210232121024 hydrogen atoms. 1 mole latex6022times1023latexatoms molecules protons etc.
 Source: cz.pinterest.com
Source: cz.pinterest.com
To transform from moles to atoms multiply the molar quantity by Avogadros quantity. 6022 10 23 atoms 1 mol or 1 mol 6022 1023 atoms Converting between Number of Moles and Number of Atoms. Type in your own numbers in the form to convert the units. This is due to the fact that the number of atoms in a mole is chosen in such a way for this to happen 602 x 1023. The Avogadros Constant is equal to 6.
 Source: pinterest.com
Source: pinterest.com
3 mole to atom 180664245E24 atom. Calculating And Converting Moles and Atoms. Atoms mols Avagadros Number 602 X 1023. Quick conversion chart of mole to atoms. 2 mole to atom 12044283E24 atom.
 Source: hu.pinterest.com
Source: hu.pinterest.com
Might 08 2018 The equation to transform moles to atoms is as follows. 5g carbon 0417 mols of carbon 12 gmol How many moles of oxygen are in 12 g of CO 2. At STP 1 mole or 602 x 1023 representative particles of any gas occupies a volume of 224 L. 1 mole to atom 60221415E23 atom. You divide and find that 1 gram of fluorine is equal to 00525350025878 moles.
 Source: pinterest.com
Source: pinterest.com
The simple unit conversion tool which helps you to convert atoms to moles or moles to atoms. 4 mole to atom 24088566E24 atom. To transform from moles to atoms multiply the molar quantity by Avogadros quantity. So there will be a total of 60210232121024 hydrogen atoms. 3 mole to atom 180664245E24 atom.
 Source: br.pinterest.com
Source: br.pinterest.com
1 mole contain 6022 140 8571023 atoms. Since water has a chemical formula of H2O there will be 2 moles of hydrogen in every mole of water. Might 08 2018 The equation to transform moles to atoms is as follows. In this video well learn to how to determine the number of atoms in one mole of a substance. We assume you are converting between mole and atom.
 Source: pinterest.com
Source: pinterest.com
Use this page to learn how to convert between moles and atoms. Use this page to learn how to convert between moles and atoms. Then you multiply that by your 878 grams. 3612 x 1023 atoms Fe. You can view more details on each measurement unit.
 Source: pinterest.com
Source: pinterest.com
4 mole to atom 24088566E24 atom. The SI base unit for amount of substance is the mole. 1 mole 60221023 6022 10 23 atoms molecules protons etc. The Mega Mole Worksheet 1-10 Convert to Moles 1204 x 1023 atoms He. To convert from moles to atoms multiply the molar amount by Avogadros number.
 Source: pinterest.com
Source: pinterest.com
B moles x 60221023 atoms 1 mole C atoms. Standard temperature and pressure STP means temperature of 0oC and a pressure of 1013 kPa or 1 atmosphere atm. That means that in a mole of water there would be one mole of oxygen and two moles of hydrogen. 1 mole latex6022times1023latexatoms molecules protons etc. This is due to the fact that the number of atoms in a mole is chosen in such a way for this to happen 602 x 1023.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to go from moles to atoms by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






