Your How to go from mole to grams images are available. How to go from mole to grams are a topic that is being searched for and liked by netizens today. You can Find and Download the How to go from mole to grams files here. Get all royalty-free images.
If you’re searching for how to go from mole to grams images information linked to the how to go from mole to grams keyword, you have come to the ideal site. Our site frequently provides you with hints for seeking the highest quality video and image content, please kindly search and locate more informative video content and graphics that fit your interests.
How To Go From Mole To Grams. Molar mass or molar weight is the mass that one mole of a substance has and they are defined in grams per mole. 7 moles in to grams 803726 gramsBased on that information to convert 1009 moles of curium to grams we multiply 1009 moles of curium by 247. Grams Moles x Molar Mass. Find the molar mass of the substance.
 Mole Conversions Made Easy How To Convert Between Grams And Moles Youtube Teaching Chemistry Mole Conversion Molar Mass From pinterest.com
Mole Conversions Made Easy How To Convert Between Grams And Moles Youtube Teaching Chemistry Mole Conversion Molar Mass From pinterest.com
Molarity x molar mass moleslitre x grams mole the moles cancel leaving grams litre. Grams Moles x Molar Mass. One mole of any substance will have 60221023 molecules of that substance. Calculate the time utilizing the present and the coulombs of cost. Converting between Liters and Moles using the Factor Label Method. Moles of given x moles needed moles given x molar mass of needed 1 mol needed 83 mol H2 x 2 mol H2O 2 mol H2 x 1802g H2O 1 mol H2O Answer.
The amount that one mole of fuel occupies at STP 224Lmol Mole to Mole Ratio.
Find the molar mass of the substance. How do you convert moles to molecules on a calculator. Successful scientists use the factor. The right way to go from moles to grams in stoichiometry. A sample of 12 grams of carbon is equal to one mole. This chemistry video tutorial explains how to convert moles to grams which is useful in typical stoichiometry problems.
 Source: pinterest.com
Source: pinterest.com
Multiply the moles given by the substances molar mass. Converting between Liters and Moles using the Factor Label Method. How to go from moles to grams. The amount of moles in a substance can be determined using that substances molar mass. Find the molar mass of the substance.
 Source: pinterest.com
Source: pinterest.com
What do we multiply moleslitre by to get gramslitre. Multiply both the values. Multiply the moles given by the substances molar mass. What do we multiply moleslitre by to get gramslitre. As explained in Introduction to Chemical Engineering.
 Source: pinterest.com
Source: pinterest.com
Tools for Today and Tomorrow 5th Edition we select that number because it is convenient and because it makes it easy to calculate how many sigmol of each substance are present in that quantity of material. This is the method of choice since you can use it to convert between any units mols to grams molecules to mols etc as long as you know the conversion factor. To go from moles to molecules multiply the number of moles by 602 x 1023. N m M the place. The amount of moles in a substance can be determined using that substances molar mass.
 Source: pinterest.com
Source: pinterest.com
N m M where. How do you convert moles to molecules on a calculator. One mole of any substance will have 60221023 molecules of that substance. This dimensional analysis video tuto. Find the molar mass of the substance.
 Source: pinterest.com
Source: pinterest.com
Formula to convert moles to grams. To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. Relating to this how is a mole ratio from a response utilized in stoichiometric issues. Select a basis of SI100gmol of exhaled gas. The molar mass is the amount of grams in one mole of a substance.
 Source: pinterest.com
Source: pinterest.com
Tools for Today and Tomorrow 5th Edition we select that number because it is convenient and because it makes it easy to calculate how many sigmol of each substance are present in that quantity of material. This is the method of choice since you can use it to convert between any units mols to grams molecules to mols etc as long as you know the conversion factor. To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams. Amazingly there are 602x1023 atoms in each of the samples above.
 Source: pinterest.com
Source: pinterest.com
Converting between Liters and Moles using the Factor Label Method. This chemistry video tutorial explains the conversion process of atoms to grams which is a typical step in common dimensional analysis stoichiometry problems. Now we are already in litres so how to convert moles to grams. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. Successful scientists use the factor.
 Source: pinterest.com
Source: pinterest.com
The right way to go from moles to grams in stoichiometry. Avogadros Number 1 mole60221023particles and the relative atomic mass of copper 1 mole of copper 63546 g are the equalities required to make this conversion. You want a formulation and a periodic desk. Multiply both the values. Molar mass or molar weight is the mass that one mole of a substance has and they are defined in grams per mole.
 Source: co.pinterest.com
Source: co.pinterest.com
To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. Note that rounding errors may occur so always check the results. In order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass. The right way to go from moles to grams in stoichiometry. Grams Moles x Molar Mass.
 Source: pinterest.com
Source: pinterest.com
Amazingly there are 602x1023 atoms in each of the samples above. What do we multiply moleslitre by to get gramslitre. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. This dimensional analysis video tuto. Convert mole fraction to mass fraction.
 Source: pinterest.com
Source: pinterest.com
A sample of 12 grams of carbon is equal to one mole. Molarity x molar mass moleslitre x grams mole the moles cancel leaving grams litre. Converting between Liters and Moles using the Factor Label Method. Calculate how many moles are mentioned in the question. Avogadros Number 1 mole60221023particles and the relative atomic mass of copper 1 mole of copper 63546 g are the equalities required to make this conversion.
 Source: pinterest.com
Source: pinterest.com
Use this page to learn how. To go from moles to molecules multiply the number of moles by 602 x 1023. Multiply the number of moles by. One mole consists of Avogadro number of atoms. Moles of given x moles needed moles given x molar mass of needed 1 mol needed 83 mol H2 x 2 mol H2O 2 mol H2 x 1802g H2O 1 mol H2O Answer.
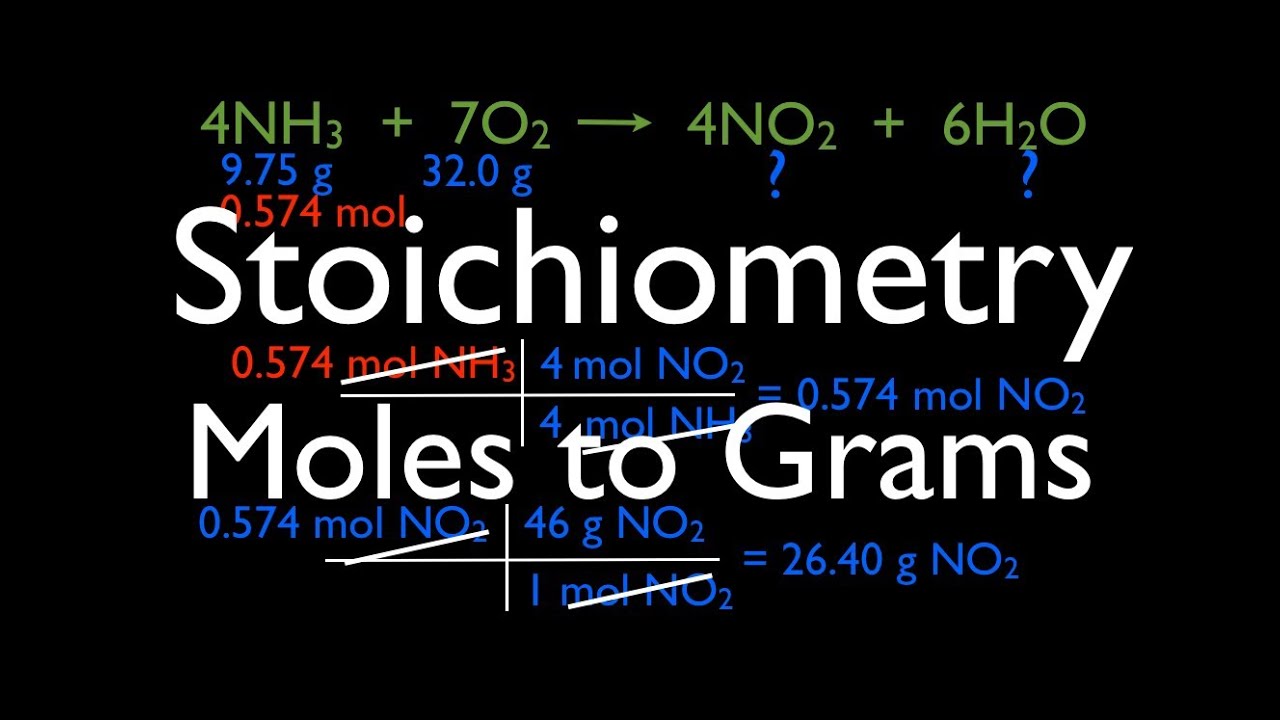 Source: pinterest.com
Source: pinterest.com
This chemistry video tutorial explains how to convert moles to grams which is useful in typical stoichiometry problems. Formula to convert moles to grams. More commonly written for this application as. This chemistry video tutorial explains how to convert moles to grams which is useful in typical stoichiometry problems. Calculate how many moles are mentioned in the question.
 Source: pinterest.com
Source: pinterest.com
This is the method of choice since you can use it to convert between any units mols to grams molecules to mols etc as long as you know the conversion factor. Molarity x molar mass moleslitre x grams mole the moles cancel leaving grams litre. Multiply both the values. Calculate how many moles are mentioned in the question. Note that rounding errors may occur so always check the results.
 Source: pinterest.com
Source: pinterest.com
As explained in Introduction to Chemical Engineering. 962 grams H 2 O x 1 mole H 2 O1802 grams 962 x 1 mole 1802 053 moles. Use this page to learn how. 02 x 5844 11688 grams. More commonly written for this application as.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to go from mole to grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






