Your How to go from mol to grams images are available. How to go from mol to grams are a topic that is being searched for and liked by netizens today. You can Find and Download the How to go from mol to grams files here. Find and Download all royalty-free images.
If you’re looking for how to go from mol to grams pictures information connected with to the how to go from mol to grams keyword, you have come to the ideal blog. Our website always gives you suggestions for seeking the maximum quality video and picture content, please kindly hunt and find more informative video content and images that match your interests.
How To Go From Mol To Grams. Molecular weight M is defined as the number of grams g per mol of a substance. The combustion of 09211 grams of MTBE C5H12Oℓ 88. Strictly speaking mol does not have dimensions of mass. For a more detailed tutorial go to Heat of Solution tutorial.
 This Is A Nice Introduction To Mole Conversions A Central Part Of Any Chemistry Class We Are Always Chemistry Lessons Teaching Chemistry Chemistry Education From pinterest.com
This Is A Nice Introduction To Mole Conversions A Central Part Of Any Chemistry Class We Are Always Chemistry Lessons Teaching Chemistry Chemistry Education From pinterest.com
H0 rxn X BEreactants X BE products C C 4 C H 3 O O 4 C O4 H O 602 kJ mol 4 413 kJ mol 3 498 kJ mol 4 799 kJ mol 4 463 kJ mol 1300 kJ mol 005 100points Methyl tert-butyl ether or MTBE is an oc-tane booster for gasoline. The combustion of 09211 grams of MTBE C5H12Oℓ 88. For a more detailed tutorial go to Heat of Solution tutorial. Molecular weight M is defined as the number of grams g per mol of a substance. Grams per mole to Kilograms per mole gmol to kgmol converter 1 Gram per mole gmol is equal 0001 Kilogram per mole kgmol use this converter Kilograms per mole to Grams per mole kgmol to gmol converter 1 Kilogram per mole kgmol is equal 1000 Grams per mole gmol use this converter. Rather mol is a primary dimension in and of itself ie the amount of matter.
H0 rxn X BEreactants X BE products C C 4 C H 3 O O 4 C O4 H O 602 kJ mol 4 413 kJ mol 3 498 kJ mol 4 799 kJ mol 4 463 kJ mol 1300 kJ mol 005 100points Methyl tert-butyl ether or MTBE is an oc-tane booster for gasoline.
Strictly speaking mol does not have dimensions of mass. H0 rxn X BEreactants X BE products C C 4 C H 3 O O 4 C O4 H O 602 kJ mol 4 413 kJ mol 3 498 kJ mol 4 799 kJ mol 4 463 kJ mol 1300 kJ mol 005 100points Methyl tert-butyl ether or MTBE is an oc-tane booster for gasoline. Note that some authors however treat mol as a unit of mass. -1 ΔH is negative because the reaction is exothermic energy is released causing the temperature of the solution to increase. For a more detailed tutorial go to Heat of Solution tutorial. The number of mols of a substance is denoted by the letter n.
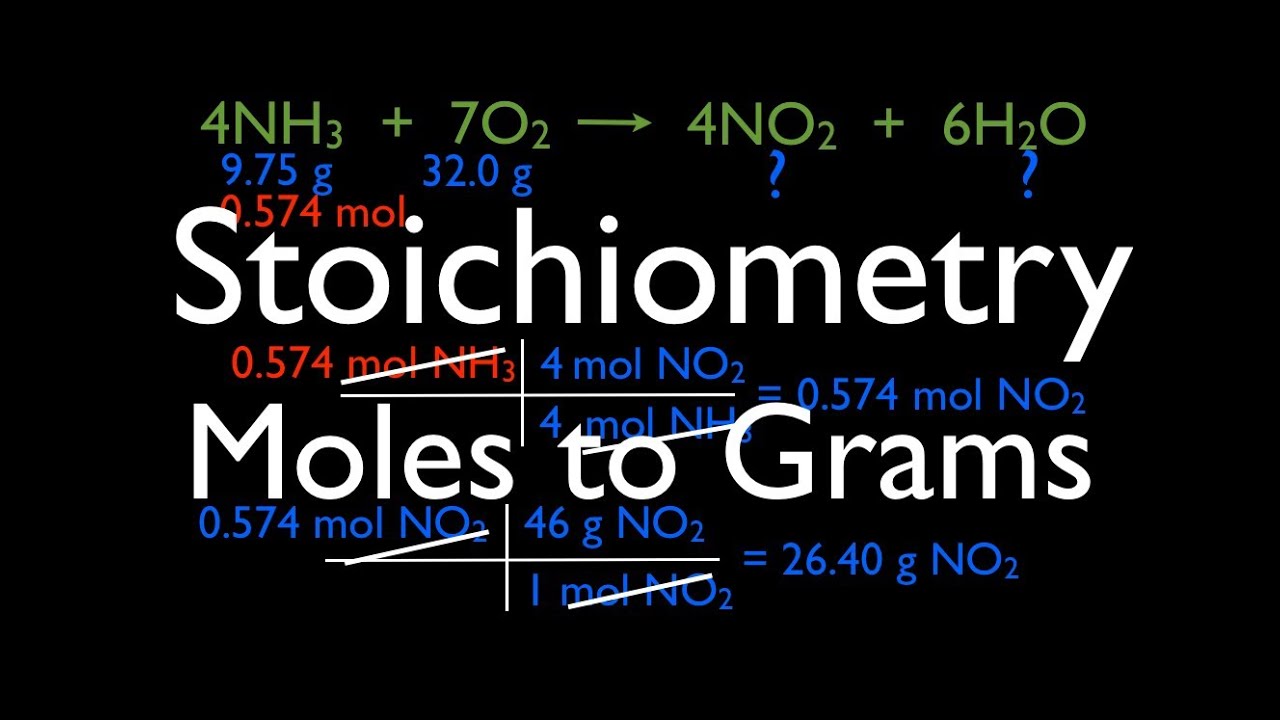 Source: pinterest.com
Source: pinterest.com
NNaOH 0030 mol Calculate the enthalpy change ΔH in kJ mol-1 of solute. H0 rxn X BEreactants X BE products C C 4 C H 3 O O 4 C O4 H O 602 kJ mol 4 413 kJ mol 3 498 kJ mol 4 799 kJ mol 4 463 kJ mol 1300 kJ mol 005 100points Methyl tert-butyl ether or MTBE is an oc-tane booster for gasoline. The number of mols of a substance is denoted by the letter n. Molecular weight M is defined as the number of grams g per mol of a substance. Grams per mole to Kilograms per mole gmol to kgmol converter 1 Gram per mole gmol is equal 0001 Kilogram per mole kgmol use this converter Kilograms per mole to Grams per mole kgmol to gmol converter 1 Kilogram per mole kgmol is equal 1000 Grams per mole gmol use this converter.
 Source: pinterest.com
Source: pinterest.com
For a more detailed tutorial go to Heat of Solution tutorial. Grams per mole to Kilograms per mole gmol to kgmol converter 1 Gram per mole gmol is equal 0001 Kilogram per mole kgmol use this converter Kilograms per mole to Grams per mole kgmol to gmol converter 1 Kilogram per mole kgmol is equal 1000 Grams per mole gmol use this converter. NNaOH 0030 mol Calculate the enthalpy change ΔH in kJ mol-1 of solute. For a more detailed tutorial go to Heat of Solution tutorial. H0 rxn X BEreactants X BE products C C 4 C H 3 O O 4 C O4 H O 602 kJ mol 4 413 kJ mol 3 498 kJ mol 4 799 kJ mol 4 463 kJ mol 1300 kJ mol 005 100points Methyl tert-butyl ether or MTBE is an oc-tane booster for gasoline.
 Source: pinterest.com
Source: pinterest.com
Molecular weight M is defined as the number of grams g per mol of a substance. -1 ΔH is negative because the reaction is exothermic energy is released causing the temperature of the solution to increase. Rather mol is a primary dimension in and of itself ie the amount of matter. Molecular weight M is defined as the number of grams g per mol of a substance. Strictly speaking mol does not have dimensions of mass.
 Source: pinterest.com
Source: pinterest.com
Molecular weight M is defined as the number of grams g per mol of a substance. Strictly speaking mol does not have dimensions of mass. For a more detailed tutorial go to Heat of Solution tutorial. Rather mol is a primary dimension in and of itself ie the amount of matter. Grams per mole to Kilograms per mole gmol to kgmol converter 1 Gram per mole gmol is equal 0001 Kilogram per mole kgmol use this converter Kilograms per mole to Grams per mole kgmol to gmol converter 1 Kilogram per mole kgmol is equal 1000 Grams per mole gmol use this converter.
 Source: pinterest.com
Source: pinterest.com
Molecular weight M is defined as the number of grams g per mol of a substance. The number of mols of a substance is denoted by the letter n. The combustion of 09211 grams of MTBE C5H12Oℓ 88. Note that some authors however treat mol as a unit of mass. Molecular weight M is defined as the number of grams g per mol of a substance.
 Source: ro.pinterest.com
Source: ro.pinterest.com
Grams per mole to Kilograms per mole gmol to kgmol converter 1 Gram per mole gmol is equal 0001 Kilogram per mole kgmol use this converter Kilograms per mole to Grams per mole kgmol to gmol converter 1 Kilogram per mole kgmol is equal 1000 Grams per mole gmol use this converter. Rather mol is a primary dimension in and of itself ie the amount of matter. H0 rxn X BEreactants X BE products C C 4 C H 3 O O 4 C O4 H O 602 kJ mol 4 413 kJ mol 3 498 kJ mol 4 799 kJ mol 4 463 kJ mol 1300 kJ mol 005 100points Methyl tert-butyl ether or MTBE is an oc-tane booster for gasoline. Molecular weight M is defined as the number of grams g per mol of a substance. For a more detailed tutorial go to Heat of Solution tutorial.
 Source: pinterest.com
Source: pinterest.com
Molecular weight M is defined as the number of grams g per mol of a substance. Rather mol is a primary dimension in and of itself ie the amount of matter. The combustion of 09211 grams of MTBE C5H12Oℓ 88. Strictly speaking mol does not have dimensions of mass. -1 ΔH is negative because the reaction is exothermic energy is released causing the temperature of the solution to increase.
 Source: pinterest.com
Source: pinterest.com
Rather mol is a primary dimension in and of itself ie the amount of matter. The number of mols of a substance is denoted by the letter n. Rather mol is a primary dimension in and of itself ie the amount of matter. For a more detailed tutorial go to Heat of Solution tutorial. H0 rxn X BEreactants X BE products C C 4 C H 3 O O 4 C O4 H O 602 kJ mol 4 413 kJ mol 3 498 kJ mol 4 799 kJ mol 4 463 kJ mol 1300 kJ mol 005 100points Methyl tert-butyl ether or MTBE is an oc-tane booster for gasoline.
 Source: pinterest.com
Source: pinterest.com
Rather mol is a primary dimension in and of itself ie the amount of matter. The number of mols of a substance is denoted by the letter n. The combustion of 09211 grams of MTBE C5H12Oℓ 88. -1 ΔH is negative because the reaction is exothermic energy is released causing the temperature of the solution to increase. Grams per mole to Kilograms per mole gmol to kgmol converter 1 Gram per mole gmol is equal 0001 Kilogram per mole kgmol use this converter Kilograms per mole to Grams per mole kgmol to gmol converter 1 Kilogram per mole kgmol is equal 1000 Grams per mole gmol use this converter.
 Source: pinterest.com
Source: pinterest.com
NNaOH 0030 mol Calculate the enthalpy change ΔH in kJ mol-1 of solute. Rather mol is a primary dimension in and of itself ie the amount of matter. Note that some authors however treat mol as a unit of mass. Grams per mole to Kilograms per mole gmol to kgmol converter 1 Gram per mole gmol is equal 0001 Kilogram per mole kgmol use this converter Kilograms per mole to Grams per mole kgmol to gmol converter 1 Kilogram per mole kgmol is equal 1000 Grams per mole gmol use this converter. -1 ΔH is negative because the reaction is exothermic energy is released causing the temperature of the solution to increase.
 Source: pinterest.com
Source: pinterest.com
-1 ΔH is negative because the reaction is exothermic energy is released causing the temperature of the solution to increase. NNaOH 0030 mol Calculate the enthalpy change ΔH in kJ mol-1 of solute. Grams per mole to Kilograms per mole gmol to kgmol converter 1 Gram per mole gmol is equal 0001 Kilogram per mole kgmol use this converter Kilograms per mole to Grams per mole kgmol to gmol converter 1 Kilogram per mole kgmol is equal 1000 Grams per mole gmol use this converter. H0 rxn X BEreactants X BE products C C 4 C H 3 O O 4 C O4 H O 602 kJ mol 4 413 kJ mol 3 498 kJ mol 4 799 kJ mol 4 463 kJ mol 1300 kJ mol 005 100points Methyl tert-butyl ether or MTBE is an oc-tane booster for gasoline. Molecular weight M is defined as the number of grams g per mol of a substance.
 Source: pinterest.com
Source: pinterest.com
NNaOH 0030 mol Calculate the enthalpy change ΔH in kJ mol-1 of solute. NNaOH 0030 mol Calculate the enthalpy change ΔH in kJ mol-1 of solute. For a more detailed tutorial go to Heat of Solution tutorial. -1 ΔH is negative because the reaction is exothermic energy is released causing the temperature of the solution to increase. H0 rxn X BEreactants X BE products C C 4 C H 3 O O 4 C O4 H O 602 kJ mol 4 413 kJ mol 3 498 kJ mol 4 799 kJ mol 4 463 kJ mol 1300 kJ mol 005 100points Methyl tert-butyl ether or MTBE is an oc-tane booster for gasoline.
 Source: pinterest.com
Source: pinterest.com
H0 rxn X BEreactants X BE products C C 4 C H 3 O O 4 C O4 H O 602 kJ mol 4 413 kJ mol 3 498 kJ mol 4 799 kJ mol 4 463 kJ mol 1300 kJ mol 005 100points Methyl tert-butyl ether or MTBE is an oc-tane booster for gasoline. Rather mol is a primary dimension in and of itself ie the amount of matter. NNaOH 0030 mol Calculate the enthalpy change ΔH in kJ mol-1 of solute. -1 ΔH is negative because the reaction is exothermic energy is released causing the temperature of the solution to increase. For a more detailed tutorial go to Heat of Solution tutorial.
 Source: pinterest.com
Source: pinterest.com
-1 ΔH is negative because the reaction is exothermic energy is released causing the temperature of the solution to increase. For a more detailed tutorial go to Heat of Solution tutorial. NNaOH 0030 mol Calculate the enthalpy change ΔH in kJ mol-1 of solute. The combustion of 09211 grams of MTBE C5H12Oℓ 88. The number of mols of a substance is denoted by the letter n.
 Source: pinterest.com
Source: pinterest.com
For a more detailed tutorial go to Heat of Solution tutorial. -1 ΔH is negative because the reaction is exothermic energy is released causing the temperature of the solution to increase. Molecular weight M is defined as the number of grams g per mol of a substance. The combustion of 09211 grams of MTBE C5H12Oℓ 88. NNaOH 0030 mol Calculate the enthalpy change ΔH in kJ mol-1 of solute.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to go from mol to grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






