Your How to go from grams to moles to atoms images are ready. How to go from grams to moles to atoms are a topic that is being searched for and liked by netizens today. You can Download the How to go from grams to moles to atoms files here. Download all free images.
If you’re looking for how to go from grams to moles to atoms images information connected with to the how to go from grams to moles to atoms topic, you have pay a visit to the ideal site. Our website always gives you hints for refferencing the maximum quality video and picture content, please kindly surf and locate more enlightening video articles and graphics that match your interests.
How To Go From Grams To Moles To Atoms. We know that the molar mass of 1 mole of carbon is equal to 12. Mols Example How many mols of are in 5g of. Note that rounding errors may occur so always check the results. 20 molesliter 05L 1mol NaCl.
 Biology Guide To Converting Moles Google Search Ensenanza De Quimica Profesor De Quimica Quimica From pinterest.com
Biology Guide To Converting Moles Google Search Ensenanza De Quimica Profesor De Quimica Quimica From pinterest.com
6022 10 23 atoms 1 mol or 1 mol 6022 1023 atoms Converting between Number of Moles and Number of Atoms. When converting grams to moles we need to know two things. How many grams of NaCl are required to make 500mL of a 20M solution. As you already know how the grams to moles conversion work find the number of moles. How to Convert Grams to Atoms. How do we set up the problem.
1000 milligrams 1 gram 1000 grams 1 kg 10 x 10 6 m g microgram 1 gram.
1000 milligrams 1 gram 1000 grams 1 kg 10 x 10 6 m g microgram 1 gram. One mole of any atom has a weight equal to the atomic mass expressed in grams. How many grams of NaCl are required to make 500mL of a 20M solution. To go from moles to molecules multiply the number of moles by 602 x 10 23. Then you multiply that by your 878 grams. Answer 1 of 2.
 Source: pinterest.com
Source: pinterest.com
How do you find atoms. As a simple example a molecule of water is made out of one hydrogen atom and two oxygen atoms. The mass of one mole of an element depends on what that element is and is equal to the atom mass of that element in grams. 1 mole is equal to 60221415E23 atom. How do we set up the problem.
 Source: pinterest.com
Source: pinterest.com
You cannot directly convert grams to atoms. Use this page to learn how to convert between moles and atoms. To convert from moles to atoms multiply the molar amount by Avogadros number. You can always use our grams to moles calculator to check the result. Note that rounding errors may occur so always check the results.
 Source: pinterest.com
Source: pinterest.com
Then you multiply that by your 878 grams. How to Convert Grams to Atoms. To go from moles to molecules multiply the number of moles by 602 x 10 23. The number of grams of the chemical. Do you not simply divide by 12.
 Source: pinterest.com
Source: pinterest.com
There is no conversion formula between the two units. Bridge type 2 1 molar mass in grams or gram molecular wt if we are using elements 1 mole. Moles Units and Moles Units and Conversion FactorsConversion Factors. To convert from atoms to moles divide the atom amount by Avogadros number or multiply by its reciprocal. How do you find atoms.
 Source: hu.pinterest.com
Source: hu.pinterest.com
This is due to the fact that the number of atoms in a mole is. One mole of any atom has a weight equal to the atomic mass expressed in grams. Also the Avogadro constant is 602 x 10 23. As you already know how the grams to moles conversion work find the number of moles. Avogadros number is a very important relationship to remember.
 Source: pinterest.com
Source: pinterest.com
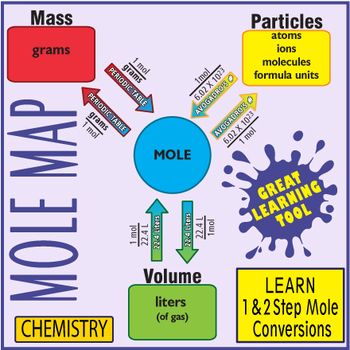
First you must covert your grams to moles then you can take the moles and covert to atoms. Look at the conversion map. Then you multiply that by your 878 grams. You cannot directly convert grams to atoms. 1000 milligrams 1 gram 1000 grams 1 kg 10 x 10 6 m g microgram 1 gram.
 Source: pinterest.com
Source: pinterest.com
As you already know how the grams to moles conversion work find the number of moles. How to Convert Grams to Atoms. Things To Remember About Moles. First box is info given next 3 boxes are the 3 conversion last box fifth box is what the question asked for. The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12.
 Source: co.pinterest.com
Source: co.pinterest.com
How to Convert Grams to Atoms. First box is info given next 3 boxes are the 3 conversion last box fifth box is what the question asked for. How do you find atoms. 1000 milligrams 1 gram 1000 grams 1 kg 10 x 10 6 m g microgram 1 gram. Converting Grams to Moles.
 Source: pinterest.com
Source: pinterest.com
1 mole 60221023 6022 10 23 atoms molecules protons etc. 12 g CH 4. So the conversion of carbon. First you must covert your grams to moles then you can take the moles and covert to atoms. 6022 10 23 atoms 1 mol or 1 mol 6022 1023 atoms Converting between Number of Moles and Number of Atoms.
 Source: pinterest.com
Source: pinterest.com
The mass of one mole of an element depends on what that element is and is equal to the atom mass of that element in grams. And this is a good example because we use the dozen as a natural counting number because we can count to 12 simply by using our thumb and the 12 finger. Quick conversion chart of mole to atom. Also the Avogadro constant is 602 x 10 23. 3 arrows 3 conversion.
 Source: pinterest.com
Source: pinterest.com
Do you not simply divide by 12. And this is a good example because we use the dozen as a natural counting number because we can count to 12 simply by using our thumb and the 12 finger. First box is info given next 3 boxes are the 3 conversion last box fifth box is what the question asked for. Atomic mass is expressed in atomic mass units while molar mass is expressed in grams. So the conversion of carbon.
 Source: pinterest.com
Source: pinterest.com
This chemistry video tutorial explains the conversion process of atoms to grams which is a typical step in common dimensional analysis stoichiometry problems. We know that the molar mass of 1 mole of carbon is equal to 12. Type in your own numbers in the form to convert the units. First box is info given next 3 boxes are the 3 conversion last box fifth box is what the question asked for. Now it should be converted in terms of atoms.
 Source: br.pinterest.com
Source: br.pinterest.com
Then you multiply that by your 878 grams. Then you multiply that by your 878 grams. This chemistry video tutorial explains the conversion process of atoms to grams which is a typical step in common dimensional analysis stoichiometry problems. Also the Avogadro constant is 602 x 10 23. How many grams of NaCl are required to make 500mL of a 20M solution.
 Source: pinterest.com
Source: pinterest.com
A mole is the quantity of a substance which possesses the same number of particles possessed by 12 grams of carbon-12. As you already know how the grams to moles conversion work find the number of moles. Now it should be converted in terms of atoms. We know that the molar mass of 1 mole of carbon is equal to 12. To go from molecules to moles divide the numbers of molecules by 602 x 10 23.
 Source: pinterest.com
Source: pinterest.com
Then you multiply that by your 878 grams. 1 mole is equal to 60221415E23 atom. Do you not simply divide by 12. Suppose you have 10 gram of carbon. Converting Grams to Moles.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to go from grams to moles to atoms by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






