Your How to get moles from molar mass and grams images are available. How to get moles from molar mass and grams are a topic that is being searched for and liked by netizens today. You can Download the How to get moles from molar mass and grams files here. Download all royalty-free images.
If you’re searching for how to get moles from molar mass and grams images information related to the how to get moles from molar mass and grams topic, you have come to the right blog. Our website always provides you with hints for downloading the maximum quality video and picture content, please kindly surf and locate more enlightening video articles and images that match your interests.
How To Get Moles From Molar Mass And Grams. There are 23811 grams of hydrogen peroxide in 0700 moles of hydrogen peroxide. And of course i the. Molar mass 34016 gramsmol. Find the molar mass of the substance.
 Converting Grams To Moles Grams To Moles Mass To Moles Mole Conversion From pinterest.com
Converting Grams To Moles Grams To Moles Mass To Moles Mole Conversion From pinterest.com
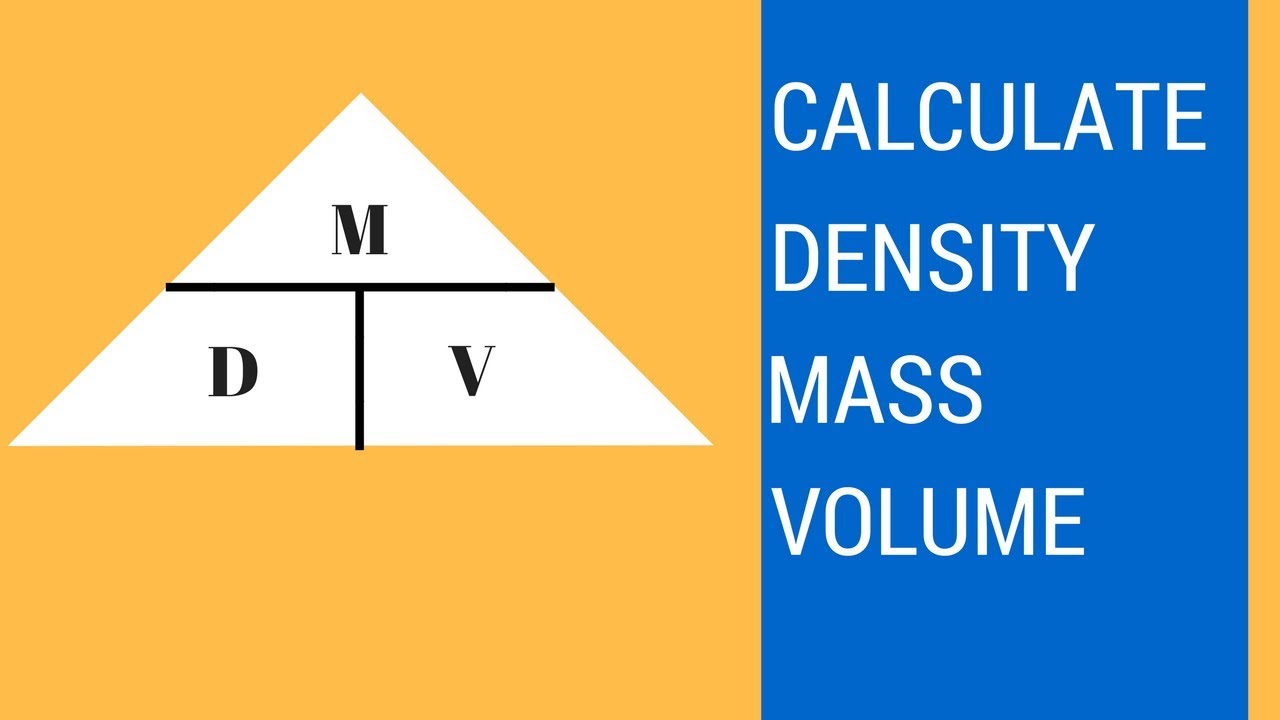
This converts atomic units to grams per mole. Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams. Now we have to perform moles to grams calculation. Here we go from a product to a reactant showing that mole-mass problems can begin and end with any. Answer 1 of 3. Formula to convert moles to grams.
By multiplying a given mass by the molar mass the.
Convert 02 moles of Sodium chloride. Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams. Calculate he number of moles you have by taking the Mass molar mass. If you know the quantity of mole it can be converted into grams and vice versa. Multiply the relative atomic mass by the molar mass constant. Then 1000 g 151001 gmol X g moles.
 Source: hu.pinterest.com
Source: hu.pinterest.com
3 moles in to grams 344454 grams. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. In order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass. The formula for moles to grams is given by. The number of grams of KClO3 will be 30637.
 Source: pinterest.com
Source: pinterest.com
This converts atomic units to grams per mole. N m M where M is the molar mass of this material. Molar mass 34016 gramsmol. You can view more details on each measurement unit. Well we have 1000 grams for every one kilogram.
 Source: pinterest.com
Source: pinterest.com
In order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass. Grams of hydrogen peroxide 34016 gramsmol x 0700 mol 23811 grams. How many grams of N 2 are needed to produce 217 mol of NH 3 when reacted according to this chemical equation. Multiply the atomic weight of each element with its number of atoms present in the compound. Grams Moles x Molar Mass.
 Source: pinterest.com
Source: pinterest.com
The molar mass of KClO3 is 122548 gmol. The symbol for carbon is c and the symbol for grams is g. Then 1000 g 151001 gmol X g moles. Multiply the atomic weight of each element with its number of atoms present in the compound. One mole consists of Avogadro number of atoms.
 Source: pinterest.com
Source: pinterest.com
Moles to Grams Conversion Formula Questions. And of course i the. View detail View more See also. Convert 02 moles of Sodium chloride. Multiply the molar mass by the number of moles to get the grams.
 Source: pinterest.com
Source: pinterest.com
Then multiply by Avogadros 6 1023 molecules per g mole. If you have 1000 grams. To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. Answer 1 of 3. Multiply the relative atomic mass by the molar mass constant.
 Source: pinterest.com
Source: pinterest.com
To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. If it is not then it can be found by looking at the. Multiply the molar mass by the number of moles to get the grams. View detail View more See also. So when you multiply these two out this is going to give you the number of grams we have of glucose which would be 1520 and if you have your mass in terms of grams you can then divide by your molar mass or you can view it as multiplying it by the moles per gram.
 Source: pinterest.com
Source: pinterest.com
If it is not then it can be found by looking at the. By multiplying a given mass by the molar mass the. Multiply the relative atomic mass by the molar mass constant. Calculate he number of moles you have by taking the Mass molar mass. Grams of hydrogen peroxide 34016 gramsmol x 0700 mol 23811 grams.
 Source: pinterest.com
Source: pinterest.com
N 2 g 3H 2 g 2NH 3 g Answer. Multiply the given number of moles 250 mol by the molar mass 122548 gmol to get the grams. Now using the molar mass of NH 3 which is 1703 gmol we get. Find out the molar mass of the substance hint. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula.
 Source: co.pinterest.com
Source: co.pinterest.com
To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. Multiply both the values. So when you multiply these two out this is going to give you the number of grams we have of glucose which would be 1520 and if you have your mass in terms of grams you can then divide by your molar mass or you can view it as multiplying it by the moles per gram. The unit is typically gmol. Determine the number of moles.
 Source: pinterest.com
Source: pinterest.com
Multiply the molar mass by the number of moles to get the grams. Now using the molar mass of NH 3 which is 1703 gmol we get. Moles to Grams Conversion Formula Questions. How many grams of N 2 are needed to produce 217 mol of NH 3 when reacted according to this chemical equation. To convert moles to g we multiply the moles by the molar mass also known as the molecular weight.
 Source: pinterest.com
Source: pinterest.com
N 2 g 3H 2 g 2NH 3 g Answer. If you have 1000 grams. Now we have to perform moles to grams calculation. And the most difficult task here is finding out the molar mass of the substance. Convert 02 moles of Sodium chloride.
 Source: pinterest.com
Source: pinterest.com
You can view more details on each measurement unit. Look for the atomic masses of hydrogen sulfur and oxygen. 3 moles in to grams 344454 grams. The symbol for carbon is c and the symbol for grams is g. There are 23811 grams of hydrogen peroxide in 0700 moles of hydrogen peroxide.
 Source: pinterest.com
Source: pinterest.com
Make use of the chemical formula to determine the number of atoms of each element in the compound. 3 moles in to grams 344454 grams. Multiply the atomic weight of each element with its number of atoms present in the compound. Now using the molar mass of NH 3 which is 1703 gmol we get. More commonly written for this application as.
 Source: pinterest.com
Source: pinterest.com
M n M m 3899 107868 m 420577332 g You can also verify the results by fetching the values in our free moles to grams calculator. N 2 g 3H 2 g 2NH 3 g Answer. Well we have 1000 grams for every one kilogram. Determine the number of moles. Where is the molar mass of the substance.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to get moles from molar mass and grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.