Your How to get moles from grams and molar mass images are ready in this website. How to get moles from grams and molar mass are a topic that is being searched for and liked by netizens today. You can Get the How to get moles from grams and molar mass files here. Find and Download all royalty-free vectors.
If you’re looking for how to get moles from grams and molar mass images information connected with to the how to get moles from grams and molar mass topic, you have visit the ideal blog. Our site frequently provides you with suggestions for viewing the highest quality video and image content, please kindly search and find more informative video content and images that fit your interests.
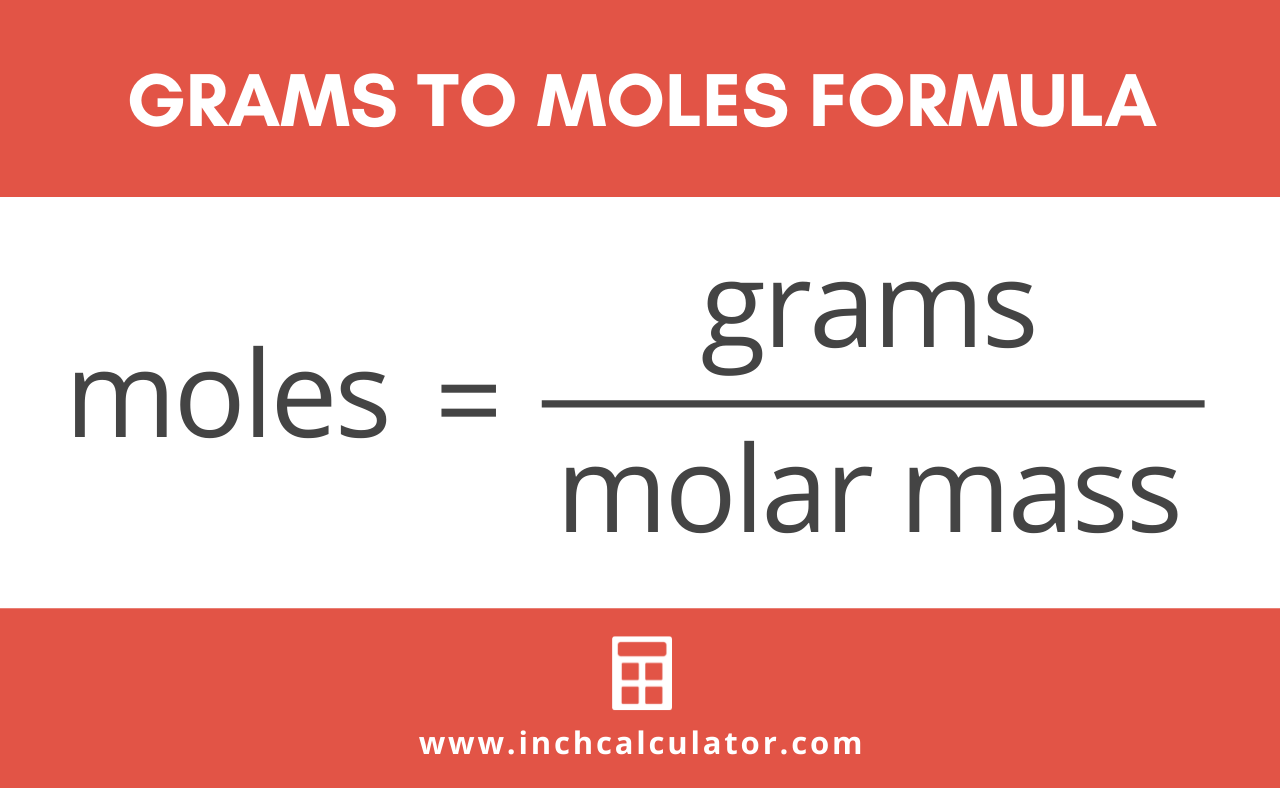
How To Get Moles From Grams And Molar Mass. Grams Moles x Molar Mass. This is defined as 0001 kilogram per mole or 1 gram per mole. Well we have 1000 grams for every one kilogram. N m M where M is the molar mass of this material.
 Chemistry Lessons Chemistry Classroom Teaching Chemistry From pinterest.com
Chemistry Lessons Chemistry Classroom Teaching Chemistry From pinterest.com
The unit is typically gmol. Finally we use the molar mass of SO 3 8006 gmol to convert to the mass of SO 3. M m n. Find the molar mass of the substance. 3 moles in to grams 344454 grams. Well we have 1000 grams for every one kilogram.
Formula to convert moles to grams.
Always multiply the subscript by the atomic mass and when its done where it equals 3198 grams. Moles to Grams Conversion Formula Questions. Oxygen has a subscript of 2 in this element and has an atomic mass of 1599 grams. More commonly written for this application as. Where is the molar mass of the substance. The number of grams of KClO3 will be 30637.
 Source: pinterest.com
Source: pinterest.com
So when you multiply these two out this is going to give you the number of grams we have of glucose which would be 1520 and if you have your mass in terms of grams you can then divide by your molar mass or you can view it as multiplying it by the moles per gram. You can view more details on each measurement unit. Grams Moles x Molar Mass. Finally we use the molar mass of SO 3 8006 gmol to convert to the mass of SO 3. More commonly written for this application as.
 Source: hu.pinterest.com
Source: hu.pinterest.com
After doing so multiply the moles with the Avogadros number. The molar mass of KClO3 is 122548 gmol. You can use Molar mass of the substance alone to calculate molar mass. Thus the molar mass of magnesium is 243050 gmol compared to carbons molar mass of 12011 gmol. For this conversion we multiply the moles to molar mass then we will get the grams.
 Source: ro.pinterest.com
Source: ro.pinterest.com
You can view more details on each measurement unit. Find the molar mass of the substance. The number of grams in the molar mass of an element is the same as the atomic mass. Hence one mole of h 2 so 4 weights 106076 grams. The mass in grams of one mole of substance is called molar mass.
 Source: pinterest.com
Source: pinterest.com
And the most difficult task here is finding out the molar mass of the substance. This converts atomic units to grams per mole. M molar mass of the pure substance measured in g mol -1 m mass of the pure substance measured in grams g. Second we use the balanced chemical reaction to convert from moles of SO 2 to moles of SO 3. Moles to Grams Conversion Formula.
 Source: pinterest.com
Source: pinterest.com
M m n. The formula for moles to grams is given by. This is defined as 0001 kilogram per mole or 1 gram per mole. Make use of the chemical formula to determine the number of atoms of each element in the compound. First of all find the number of moles by using mass and molar mass.
 Source: pinterest.com
Source: pinterest.com
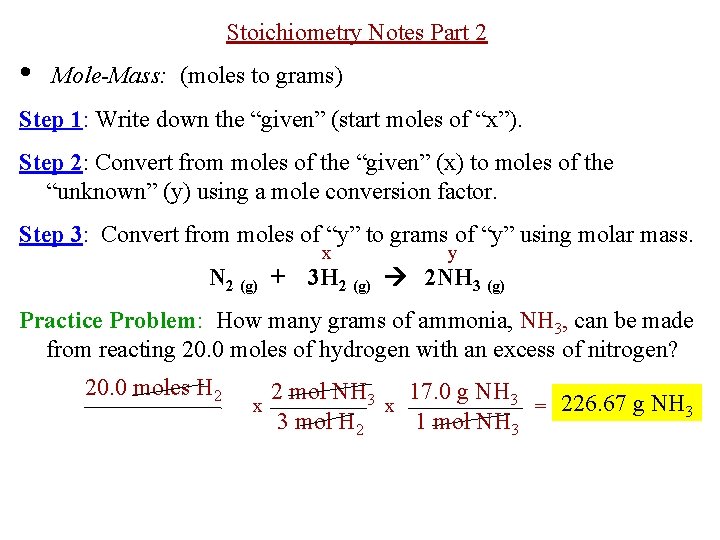
First we convert the given amount 453 g of SO 2 to moles of SO 2 using its molar mass 6406 gmol. First we convert the given amount 453 g of SO 2 to moles of SO 2 using its molar mass 6406 gmol. The formula for moles to grams is given by. In other words we can say that it is a product of moles and molar mass. Moles To Grams Calculator Formula.
 Source: pinterest.com
Source: pinterest.com
Find the molar mass of the substance. Hence one mole of h 2 so 4 weights 106076 grams. Look for the atomic masses of hydrogen sulfur and oxygen. The number of grams in the molar mass of an element is the same as the atomic mass. Oxygen has a subscript of 2 in this element and has an atomic mass of 1599 grams.
 Source: pinterest.com
Source: pinterest.com
Well we have 1000 grams for every one kilogram. In other words we can say that it is a product of moles and molar mass. To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. And the most difficult task here is finding out the molar mass of the substance. View 30 Molar Mass and Grams1docx from PHYS 1010 at University Of Georgia.
 Source: pinterest.com
Source: pinterest.com
Second we use the balanced chemical reaction to convert from moles of SO 2 to moles of SO 3. Look for the atomic masses of hydrogen sulfur and oxygen. How many moles are in 15 grams of lithium. For this conversion we multiply the moles to molar mass then we will get the grams. Grams Moles Molar mass Example.
 Source: pinterest.com
Source: pinterest.com
More commonly written for this application as. Find the molar mass of the substance. Determine the number of moles. Multiply the number of moles by the molar mass to obtain the final answer in grams. The relationship between molar mass mass and moles can be expressed as a mathematical equation as shown below.
 Source: pinterest.com
Source: pinterest.com
For this conversion we multiply the moles to molar mass then we will get the grams. Finally we use the molar mass of SO 3 8006 gmol to convert to the mass of SO 3. The number of grams in the molar mass of an element is the same as the atomic mass. Hence one mole of h 2 so 4 weights 106076 grams. This is defined as 0001 kilogram per mole or 1 gram per mole.
 Source: pinterest.com
Source: pinterest.com
The number of grams in the molar mass of an element is the same as the atomic mass. You can view more details on each measurement unit. More commonly written for this application as. Oxygen has a subscript of 2 in this element and has an atomic mass of 1599 grams. M molar mass of the pure substance measured in g mol -1 m mass of the pure substance measured in grams g.
 Source: co.pinterest.com
Source: co.pinterest.com
The unit is typically gmol. The symbol for carbon is c and the symbol for grams is g. And the most difficult task here is finding out the molar mass of the substance. How many moles are in 15 grams of lithium. Multiply the relative atomic mass by the molar mass constant.
 Source: pinterest.com
Source: pinterest.com
Moles To Grams Calculator Formula. N m M where M is the molar mass of this material. The mass in grams of one mole of substance is called molar mass. G mol -1 g mol. Hence one mole of h 2 so 4 weights 106076 grams.
 Source: pinterest.com
Source: pinterest.com
Moles to Grams Conversion Formula. Hence one mole of h 2 so 4 weights 106076 grams. You can use Molar mass of the substance alone to calculate molar mass. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. G mol -1 g mol.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to get moles from grams and molar mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.