Your How to find unknown molar mass images are ready in this website. How to find unknown molar mass are a topic that is being searched for and liked by netizens today. You can Download the How to find unknown molar mass files here. Find and Download all royalty-free photos.
If you’re looking for how to find unknown molar mass images information related to the how to find unknown molar mass interest, you have visit the right blog. Our site frequently provides you with hints for seeking the maximum quality video and picture content, please kindly surf and find more informative video content and images that fit your interests.
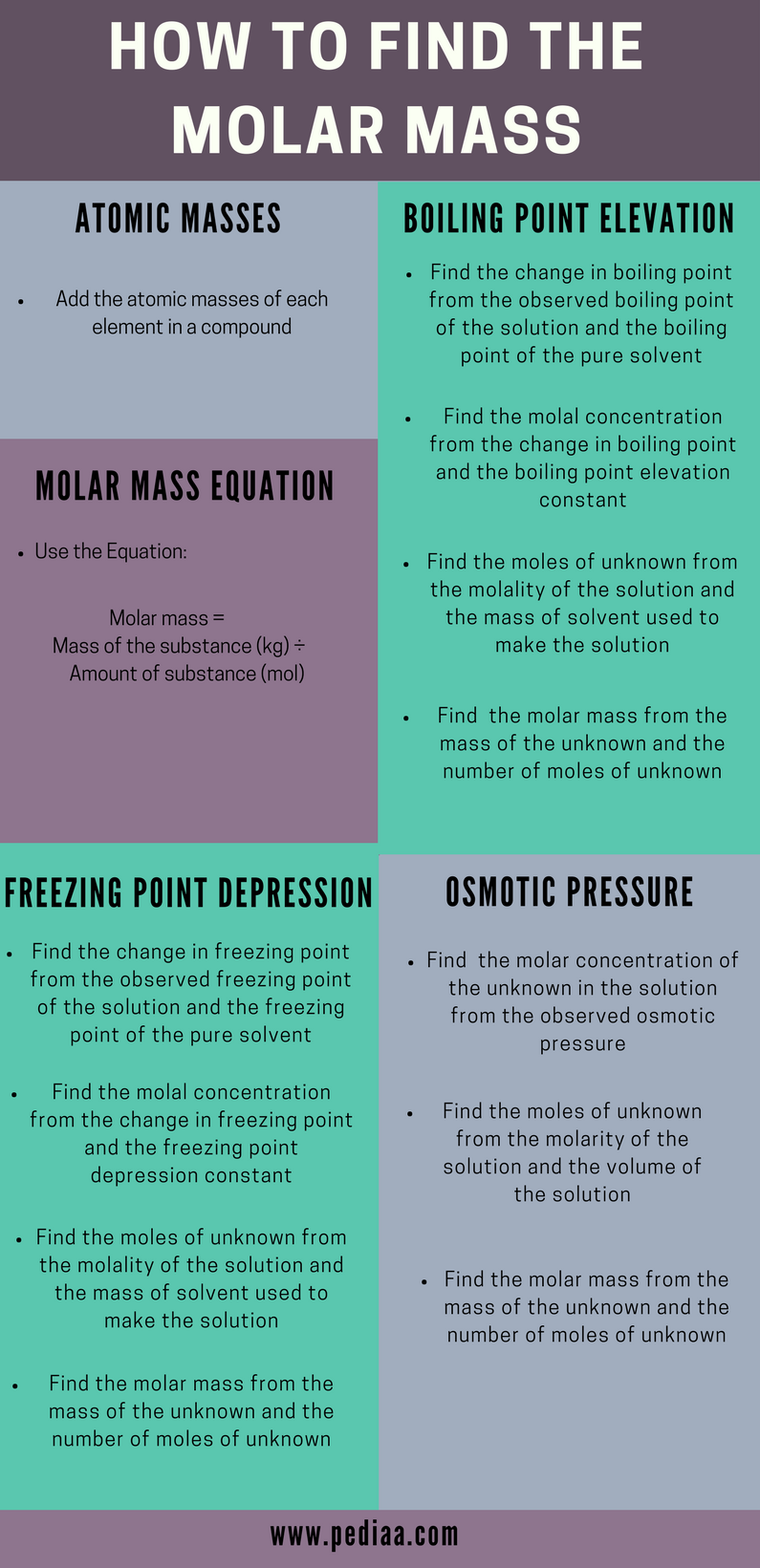
How To Find Unknown Molar Mass. Calculate the molecular formula of the compound. Determine the molar mass from the mass of the unknown and thenumber of moles of unknown. Determine the molar concentration of the unknown in the solutionfrom the observed osmotic pressure. The molar mass of a compound can be calculated by adding the standard atomic masses in gmol of the constituent atoms.
 Molar Mass From Osmotic Pressure Molarity Van T Hoff Factor Chemistry Problems Youtube From youtube.com
Molar Mass From Osmotic Pressure Molarity Van T Hoff Factor Chemistry Problems Youtube From youtube.com
Calculating the molar mass from the boiling point elevation 357 People Used More Info. List the known quantities and plan the problem. I also know the amount used of each solution Acid and Base during the titration In mL. Use the freeing point depression to calculate the molality of the solution. Molecular Weight fracWeightMoles_acid fracWeight2 times Moles_NaOH fracWeight2 times Molarity_NaOH times Volume_NaOH fraca2 times 0120 times frac2201000. Lets assume you have weighed a gram of acid.
1000g benzene x 1kg1000g 01000kg.
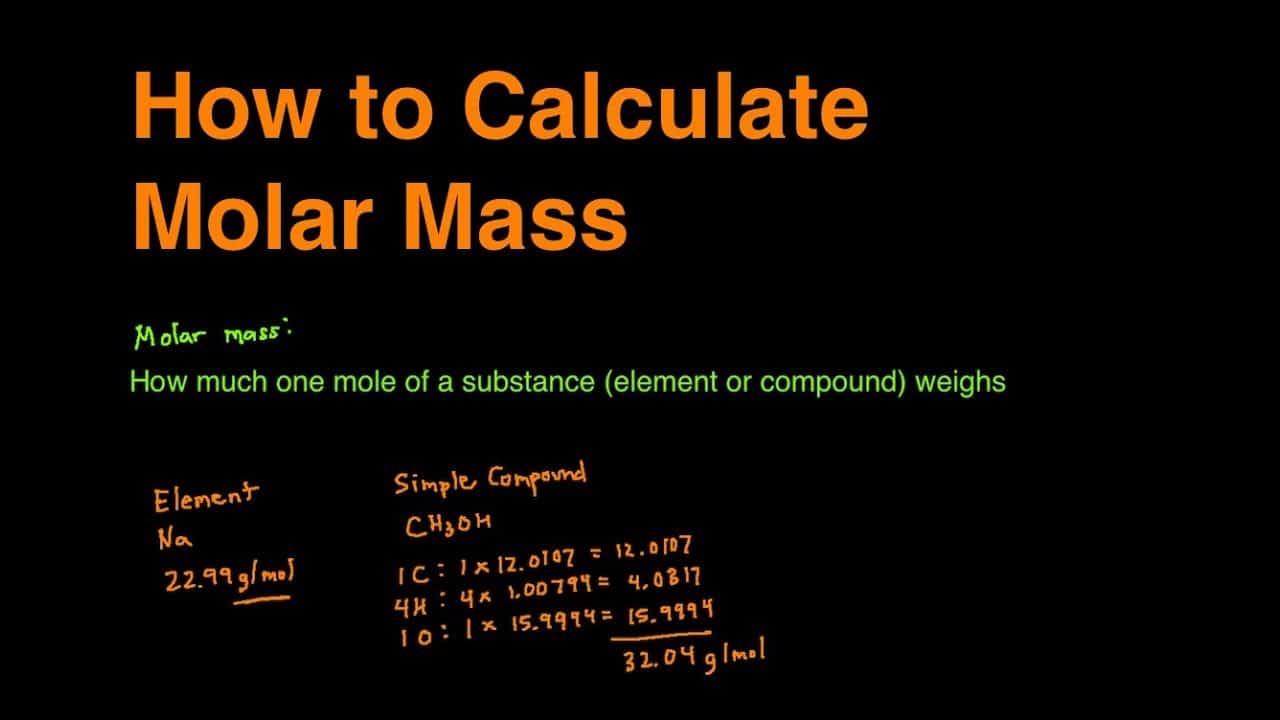
The rest here on out was a little algebra. For example the molar mass of NaCl can be calculated for finding the atomic mass of sodium 2299 gmol and the atomic mass of chlorine 3545 gmol and combining them. To find the molar mass of the metal carbonate we took the mass of the sample used the metal carbonate and divided it by moles of C O X 2 released which was just calculated. List the known quantities and plan the problem. Calculate the molar mass of the compound. M 0917m 0917molkg.
 Source: pediaa.com
Source: pediaa.com
Using the equation to calculate the molar mass. Add half of the acid to a clean. How do you find the molar mass of an unknown acid if you know the weight of the sample in grams and the volume of the solution 250mL. M 0917m 0917molkg. Obtain from your TA an unknown acid sample vial.
 Source: youtube.com
Source: youtube.com
Using atomic masses to calculate the molar mass. How do you find the molar mass of an unknown compound. Ppressure 0954 atm use kpa since that is what is required in the ideal gas law thus pressure is 0954101325 kpa 9666405 kpa Vvolume 0461 L RUniversal gas constant or 8314. Calculate the molar mass of the compound. Then divide the grams of solute by the moles to determine the molar mass.
 Source: geteducationcrunch.com
Source: geteducationcrunch.com
Write your unknown number in your notebook. Then use the molality equation to calculate the moles of solute. How do you find the molar mass of an unknown compound. MM solute m solute n solute 5 You will be working with cyclohexane as your solvent. Obtain from your TA an unknown acid sample vial.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Mass solute 387 g. Freezing point depression provides a convenient way to determine the molar mass of an unknown substance. A solution containing a known mass of solute per mass of solvent is prepared and its freezing point is measured. Titration reactions are just neutralization reactions. You may have missed the mass of the solid acid you have weighed.
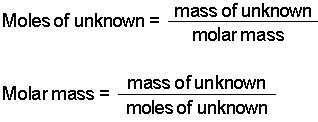 Source: chem.purdue.edu
Source: chem.purdue.edu
How do you find the molar mass of an unknown acid if you know the weight of the sample in grams and the volume of the solution 250mL. This sample vial contains two samples of your unknown acid. Number of moles Molarity X Volume in L or given mass Molar mass Hence 22871000 X 01075 03 Molar mass Thus Molar mass 12202 gmole. The moles of base titrant can be determined from the molarity of the base solution multiplied by the volume of titrant required to reach the equivalence point or moles base M. Mass solute 387 g.
 Source: youtube.com
Source: youtube.com
MM solute m solute n solute 5 You will be working with cyclohexane as your solvent. The moles of base titrant can be determined from the molarity of the base solution multiplied by the volume of titrant required to reach the equivalence point or moles base M. 4 you can determine the molar mass of the unknown solute using the equation below. Therefore we will calculate the molar mass of unknown as follows. Reaction is known the molar mass of the unknown acid can be calculated by modification of equation 3.
 Source: kentchemistry.com
Source: kentchemistry.com
Ppressure 0954 atm use kpa since that is what is required in the ideal gas law thus pressure is 0954101325 kpa 9666405 kpa Vvolume 0461 L RUniversal gas constant or 8314. List the known quantities and plan the problem. Lets assume you have weighed a gram of acid. The molar mass of a compound can be calculated by adding the standard atomic masses in gmol of the constituent atoms. Determine the moles of unknown the solute from the molarityof the solution and the volume in liters of the solution.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Find the moles of solute from molality by multiplying by the kg of solvent. Titration reactions are just neutralization reactions. Option e is the correct answer. M 0917m 0917molkg. My base is NaOH.
 Source: youtube.com
Source: youtube.com
Then use the molality equation to calculate the moles of solute. Reaction is known the molar mass of the unknown acid can be calculated by modification of equation 3. Determine the moles of unknown the solute from the molarityof the solution and the volume in liters of the solution. Weigh the vial and all its contents. How do you find the molar mass of an unknown acid if you know the weight of the sample in grams and the volume of the solution 250mL.
 Source: youtube.com
Source: youtube.com
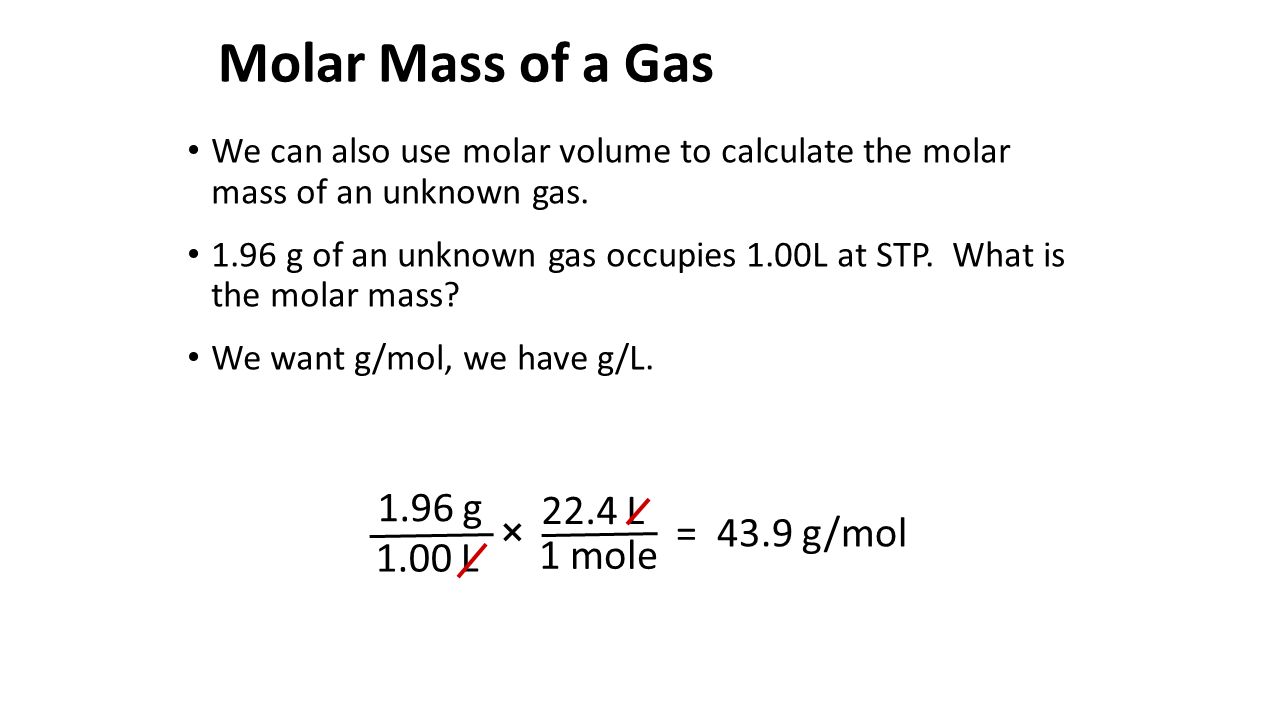
Then divide the grams of solute by the moles to determine the molar mass. Find the moles of solute from molality by multiplying by the kg of solvent. The molecular mass of the gas is 900 u. MM solute m solute n solute 5 You will be working with cyclohexane as your solvent. Therefore we will calculate the molar mass of unknown as follows.
 Source: khanacademy.org
Source: khanacademy.org
Then use the molality equation to calculate the moles of solute. Write your unknown number in your notebook. To calculate the molar mass of a compound with multiple atoms sum all the atomic mass of the constituent atoms. Determine the molar mass from the mass of the unknown and thenumber of moles of unknown. The empirical formula mass of CH2O is 3003 u.
 Source: youtube.com
Source: youtube.com
Calculate the molar mass of the compound. According to Grahams law time taken for effusion is directly proportional to square root of molar mass. 0917molkg x 01000kg 00917 moles of solute. A solution containing a known mass of solute per mass of solvent is prepared and its freezing point is measured. To calculate the molality of the solution.
 Source: slideplayer.com
Source: slideplayer.com
M solvent kg and the same value of Kf for other solutes. Mass H 2 O 218 g 0218 kg. Molecular Weight fracWeightMoles_acid fracWeight2 times Moles_NaOH fracWeight2 times Molarity_NaOH times Volume_NaOH fraca2 times 0120 times frac2201000. Therefore we will calculate the molar mass of unknown as follows. To find the molar mass of the metal carbonate we took the mass of the sample used the metal carbonate and divided it by moles of C O X 2 released which was just calculated.
 Source: geteducationcrunch.com
Source: geteducationcrunch.com
Titration reactions are just neutralization reactions. 0917molkg x 01000kg 00917 moles of solute. Calculate the molecular formula of the compound. Using the equation to calculate the molar mass. Titrations are used todetermine the amount of one substance presentby reacting it with a known amount of another substance.
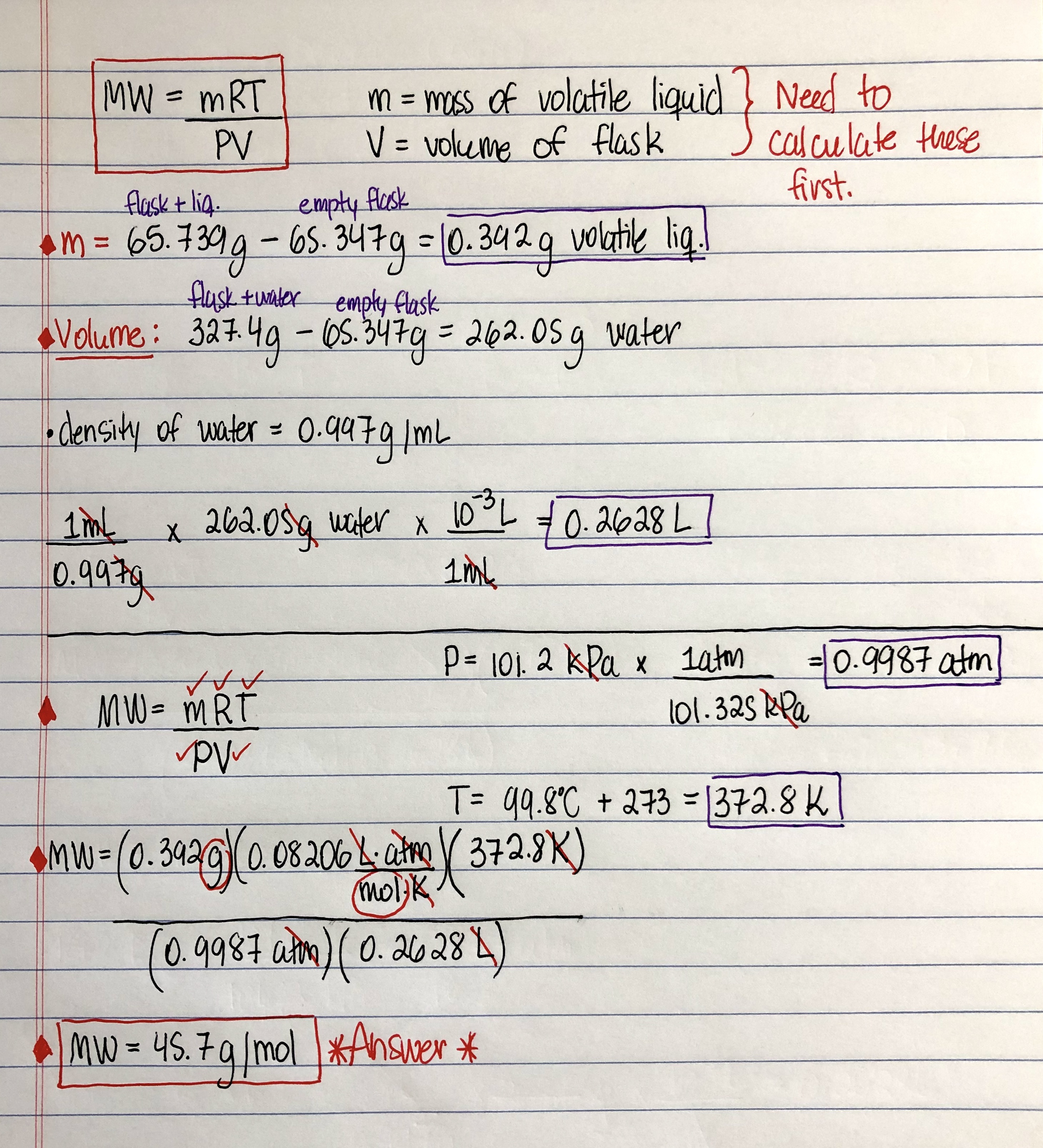 Source: clutchprep.com
Source: clutchprep.com
Calculate the molality using the change in boiling point and the elevation constant. To find the molar mass of the metal carbonate we took the mass of the sample used the metal carbonate and divided it by moles of C O X 2 released which was just calculated. The molar mass of a compound can be calculated by adding the standard atomic masses in gmol of the constituent atoms. MM solute m solute n solute 5 You will be working with cyclohexane as your solvent. How to use proportions to find the molar mass of an unknown element.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find unknown molar mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






