Your How to find the number of moles of an atom in a compound images are available in this site. How to find the number of moles of an atom in a compound are a topic that is being searched for and liked by netizens today. You can Download the How to find the number of moles of an atom in a compound files here. Find and Download all free photos and vectors.
If you’re searching for how to find the number of moles of an atom in a compound pictures information linked to the how to find the number of moles of an atom in a compound interest, you have come to the right site. Our website always gives you hints for seeing the highest quality video and image content, please kindly surf and find more informative video content and images that fit your interests.
How To Find The Number Of Moles Of An Atom In A Compound. One gram of helium for example is 14 mole. Just as the word dozen refers to the number 12 the word mole refers to Avogadros number 6021023. You can work with fractions of moles. Using the chemical equation you can identify the atoms of each element in the reaction.
 Calculate The Number Of Atoms In 0 5 Mole Atoms Of Nitrogen Youtube From youtube.com
Calculate The Number Of Atoms In 0 5 Mole Atoms Of Nitrogen Youtube From youtube.com
You can work with fractions of moles. Using the chemical equation you can identify the atoms of each element in the reaction. Because a chemical reaction can never create or destroy new matter a given equation is unbalanced if the number and types of atoms on each side of the equation dont perfectly. A mole represents a number. To find the number of atoms in one gram of helium multiply 6021023 by 14. Just as the word dozen refers to the number 12 the word mole refers to Avogadros number 6021023.
To find the number of atoms in one gram of helium multiply 6021023 by 14.
To find the number of atoms in one gram of helium multiply 6021023 by 14. Because a chemical reaction can never create or destroy new matter a given equation is unbalanced if the number and types of atoms on each side of the equation dont perfectly. Write down the number of atoms that comprise each compound on either side of the equation. Just as the word dozen refers to the number 12 the word mole refers to Avogadros number 6021023. One gram of helium for example is 14 mole. You can work with fractions of moles.
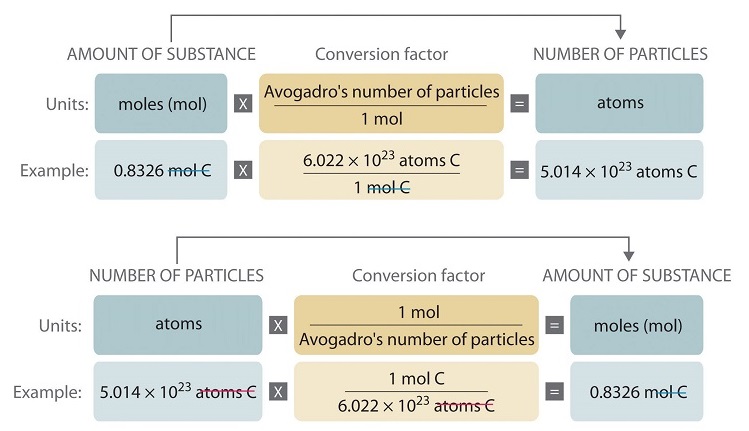 Source: chem.libretexts.org
Source: chem.libretexts.org
Using the chemical equation you can identify the atoms of each element in the reaction. A mole represents a number. To find the number of atoms in one gram of helium multiply 6021023 by 14. Using the chemical equation you can identify the atoms of each element in the reaction. Because a chemical reaction can never create or destroy new matter a given equation is unbalanced if the number and types of atoms on each side of the equation dont perfectly.
 Source: youtube.com
Source: youtube.com
One gram of helium for example is 14 mole. A mole represents a number. Because a chemical reaction can never create or destroy new matter a given equation is unbalanced if the number and types of atoms on each side of the equation dont perfectly. One gram of helium for example is 14 mole. You can work with fractions of moles.
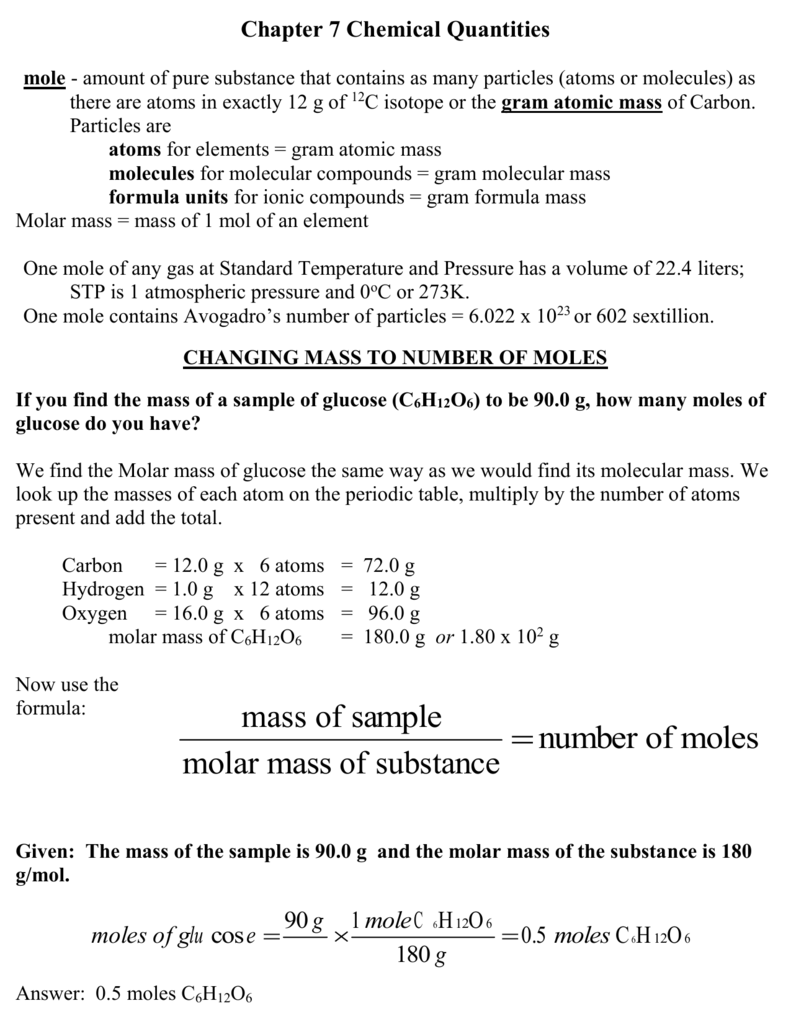 Source: studylib.net
Source: studylib.net
Because a chemical reaction can never create or destroy new matter a given equation is unbalanced if the number and types of atoms on each side of the equation dont perfectly. To find the number of atoms in one gram of helium multiply 6021023 by 14. You can work with fractions of moles. Because a chemical reaction can never create or destroy new matter a given equation is unbalanced if the number and types of atoms on each side of the equation dont perfectly. Write down the number of atoms that comprise each compound on either side of the equation.
 Source: chemistry.wustl.edu
Source: chemistry.wustl.edu
You can work with fractions of moles. You can work with fractions of moles. Using the chemical equation you can identify the atoms of each element in the reaction. A mole represents a number. Because a chemical reaction can never create or destroy new matter a given equation is unbalanced if the number and types of atoms on each side of the equation dont perfectly.
 Source: youtube.com
Source: youtube.com
A mole represents a number. One gram of helium for example is 14 mole. Just as the word dozen refers to the number 12 the word mole refers to Avogadros number 6021023. A mole represents a number. Because a chemical reaction can never create or destroy new matter a given equation is unbalanced if the number and types of atoms on each side of the equation dont perfectly.
 Source: youtube.com
Source: youtube.com
Using the chemical equation you can identify the atoms of each element in the reaction. You can work with fractions of moles. Using the chemical equation you can identify the atoms of each element in the reaction. Just as the word dozen refers to the number 12 the word mole refers to Avogadros number 6021023. One gram of helium for example is 14 mole.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Just as the word dozen refers to the number 12 the word mole refers to Avogadros number 6021023. Just as the word dozen refers to the number 12 the word mole refers to Avogadros number 6021023. To find the number of atoms in one gram of helium multiply 6021023 by 14. Using the chemical equation you can identify the atoms of each element in the reaction. Write down the number of atoms that comprise each compound on either side of the equation.
 Source: youtube.com
Source: youtube.com
To find the number of atoms in one gram of helium multiply 6021023 by 14. Using the chemical equation you can identify the atoms of each element in the reaction. Just as the word dozen refers to the number 12 the word mole refers to Avogadros number 6021023. One gram of helium for example is 14 mole. Because a chemical reaction can never create or destroy new matter a given equation is unbalanced if the number and types of atoms on each side of the equation dont perfectly.
 Source: slideplayer.com
Source: slideplayer.com
Using the chemical equation you can identify the atoms of each element in the reaction. A mole represents a number. To find the number of atoms in one gram of helium multiply 6021023 by 14. Write down the number of atoms that comprise each compound on either side of the equation. You can work with fractions of moles.
 Source: khanacademy.org
Source: khanacademy.org
To find the number of atoms in one gram of helium multiply 6021023 by 14. Just as the word dozen refers to the number 12 the word mole refers to Avogadros number 6021023. You can work with fractions of moles. Using the chemical equation you can identify the atoms of each element in the reaction. A mole represents a number.
 Source: slideshare.net
Source: slideshare.net
Just as the word dozen refers to the number 12 the word mole refers to Avogadros number 6021023. You can work with fractions of moles. One gram of helium for example is 14 mole. Because a chemical reaction can never create or destroy new matter a given equation is unbalanced if the number and types of atoms on each side of the equation dont perfectly. Using the chemical equation you can identify the atoms of each element in the reaction.
 Source: thefactfactor.com
Source: thefactfactor.com
Just as the word dozen refers to the number 12 the word mole refers to Avogadros number 6021023. Write down the number of atoms that comprise each compound on either side of the equation. Just as the word dozen refers to the number 12 the word mole refers to Avogadros number 6021023. Because a chemical reaction can never create or destroy new matter a given equation is unbalanced if the number and types of atoms on each side of the equation dont perfectly. One gram of helium for example is 14 mole.
 Source: youtube.com
Source: youtube.com
Using the chemical equation you can identify the atoms of each element in the reaction. Write down the number of atoms that comprise each compound on either side of the equation. You can work with fractions of moles. One gram of helium for example is 14 mole. A mole represents a number.
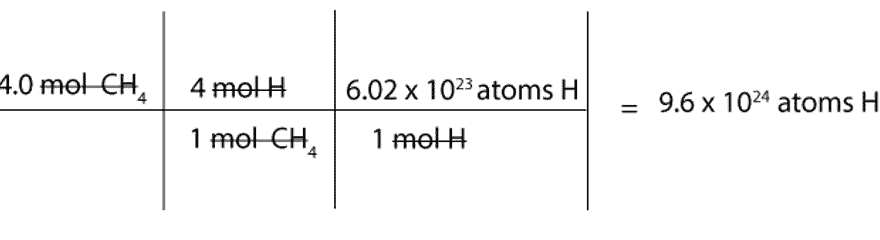 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
Write down the number of atoms that comprise each compound on either side of the equation. Because a chemical reaction can never create or destroy new matter a given equation is unbalanced if the number and types of atoms on each side of the equation dont perfectly. You can work with fractions of moles. A mole represents a number. Just as the word dozen refers to the number 12 the word mole refers to Avogadros number 6021023.
 Source: youtube.com
Source: youtube.com
Just as the word dozen refers to the number 12 the word mole refers to Avogadros number 6021023. To find the number of atoms in one gram of helium multiply 6021023 by 14. Just as the word dozen refers to the number 12 the word mole refers to Avogadros number 6021023. Write down the number of atoms that comprise each compound on either side of the equation. A mole represents a number.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find the number of moles of an atom in a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






