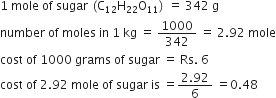Your How to find the moles of ions in a compound images are available. How to find the moles of ions in a compound are a topic that is being searched for and liked by netizens today. You can Find and Download the How to find the moles of ions in a compound files here. Find and Download all free images.
If you’re looking for how to find the moles of ions in a compound pictures information related to the how to find the moles of ions in a compound interest, you have come to the right blog. Our website always provides you with hints for refferencing the highest quality video and image content, please kindly surf and find more enlightening video content and graphics that match your interests.
How To Find The Moles Of Ions In A Compound. The mathematical equation N n N A can also be used to find the number of atoms of each element in a known amount in moles of a compound. The oxidation number of A is 1 the oxidation number of B is 2 the oxidation number of C is 3 and the oxidation number of D is 0. Atomic mass of Cu 6355. The compound calcium chloride is a giant lattice of calcium and chloride ions.
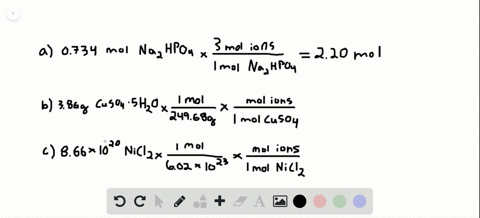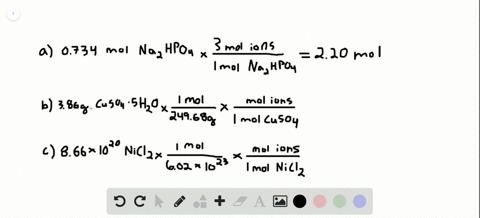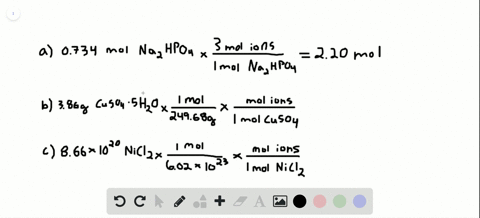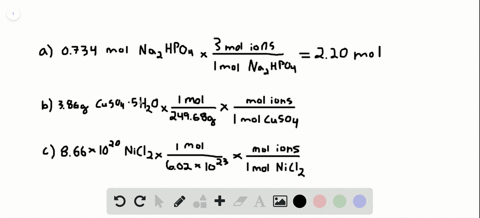
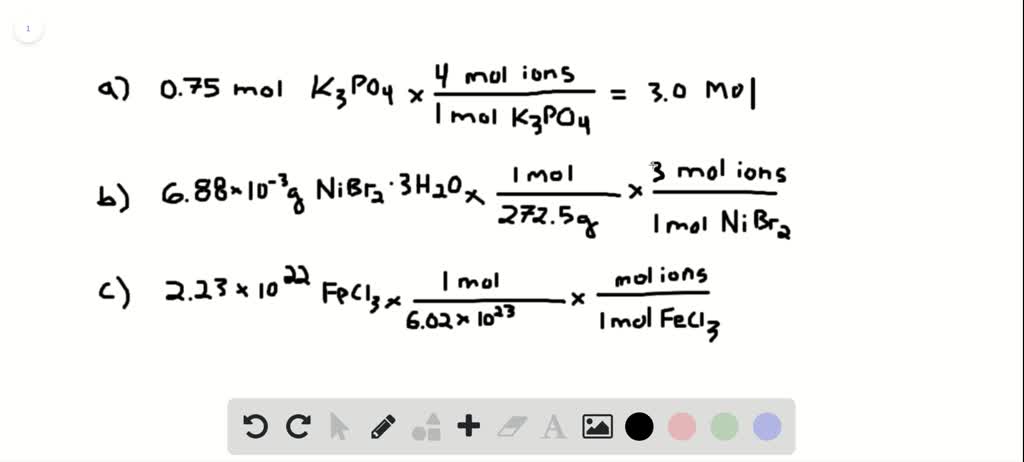 Solved How Many Total Moles Of Ions Are Released When Each Of The Following Dissolves In Water A 0 75 Mol Of Mathrm K 3 Mathrm Po 4 Quad B 6 88 Times 10 3 Mathrm G Of Mathrm Nibr 2 Cdot 3 From numerade.com
Solved How Many Total Moles Of Ions Are Released When Each Of The Following Dissolves In Water A 0 75 Mol Of Mathrm K 3 Mathrm Po 4 Quad B 6 88 Times 10 3 Mathrm G Of Mathrm Nibr 2 Cdot 3 From numerade.com
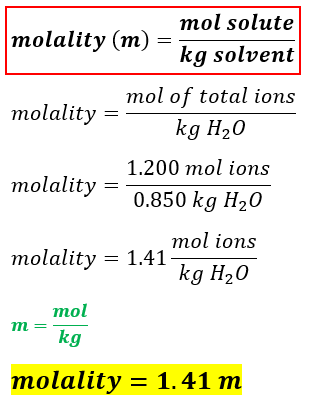
For every mole of MgI2 there is one mole of Mg and 2 moles of I-. So given 25 moles of CaCl2 when dissolved in a suitable solvent will give you 25 moles of Clacium ions 1x2. The mathematical equation N n N A can also be used to find the number of atoms of each element in a known amount in moles of a compound. 1 mole atoms 60221023 atoms. We now know that in every mole of adrenaline there are 48 mathrmg of oxygen. Furthermore you also need to know the molar mass of the solute.
O - 300495 mol 0148 mol.
The number of the constituent particles of atoms such as molecules or atoms or ions present in a. N 1 mole of N for 1 mole of KNO3. If the focus of Na ion on this resolution is 02 molar discover x within the components of compound. So the correct answer is a. Use stoichiometry and dimensional analysis to calculate the number of atoms in 5 moles of NaOH. Calculate Percentage Composition of Oxygen by Mass.
 Source: youtube.com
Source: youtube.com
Execute I require to obtain the number mole of every ion current from the calculated total variety of moles because that the molecule. N the number of moles of a compound. 2K and SO42- ions. To acquire the variety of moles divide the mass of compound by the molar mass of the compound expressed in grams. Atomic mass of Cu 6355.
 Source: youtube.com
Source: youtube.com
We now know that in every mole of adrenaline there are 48 mathrmg of oxygen. Consequently how many moles of chloride ions are there in 2 moles of calcium chloride. Furthermore you also need to know the molar mass of the solute. Lets start with these values. Number of ions left textnumber of moles righttextX left N_A right where N_A Avogadros number.
 Source: numerade.com
Source: numerade.com
To acquire the variety of moles divide the mass of compound by the molar mass of the compound expressed in grams. Be able to calculate the moles of a compound if given the mass and molar mass or if given the molarity and volume know when to convert from mL to L note that molarity is in molesL Divide the mass of the substance g by the molar mass of the element. So the correct answer is a. So in each formula unit there are 3 sodium ions and 1 phosphate ion. The mathematical equation N n N A can also be used to find the number of atoms of each element in a known amount in moles of a compound.
 Source: clutchprep.com
Source: clutchprep.com
00035 grams x 1 mole 2781 grams moles of MgI2. How to find moles in the solution is to calculate how many molecules the solution contains. 00800 mol A Moles of B in 100 grams of the compound. Find the molarity of the solute. O - 300495 mol 0148 mol.
 Source: youtube.com
Source: youtube.com
Most noteworthy every molecule has 1 Na Sodium and 1 Cl Chloride atom. 10 moles of sodium ions 10 6022 10 23 6022 10 24 sodium ions. We also know that 1 mathrmmol of adrenaline weighs 183 mathrmg. Moles of element n moles elementone mole of compound K - 1 mole of K for 1 mole of KNO3. Multiply the concentration in M times Avogadros Number time 3 to get the number of ions from the sodium bicarbonate.
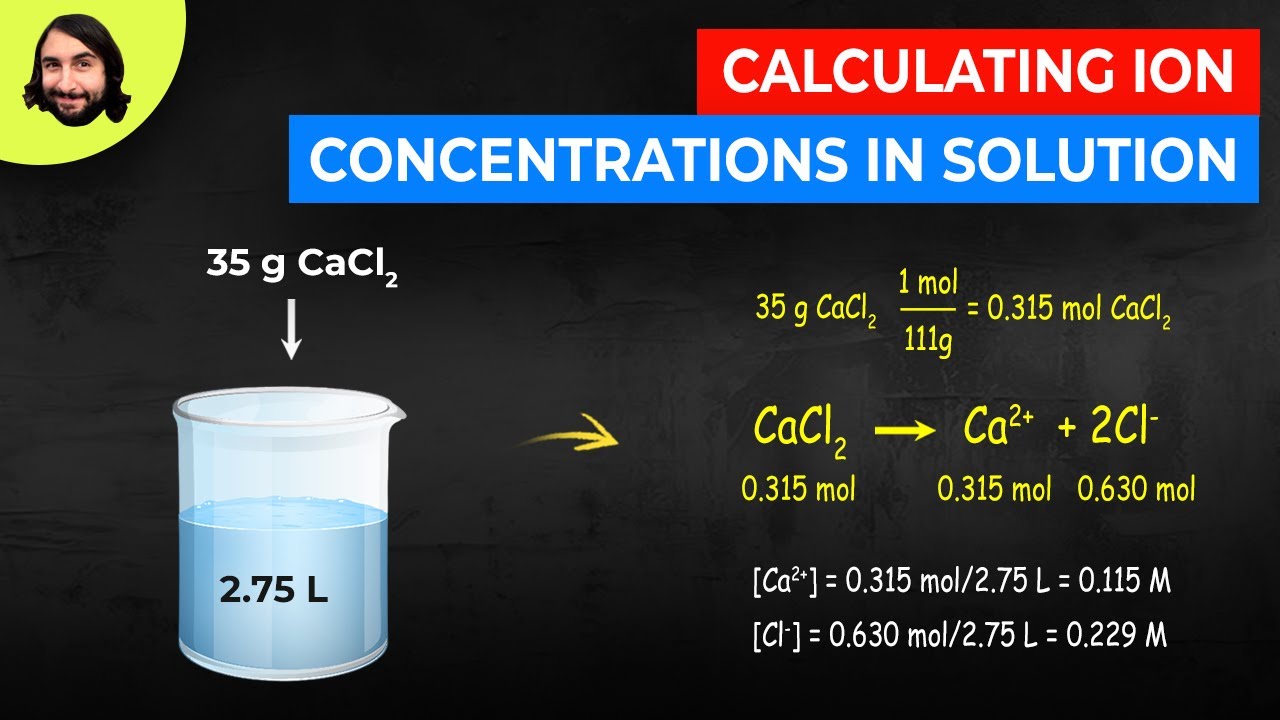 Source: youtube.com
Source: youtube.com
Yes exactly there space three ions present so every you should do is multiply 0599 through three and you will have your last answer. First we need to get the mass of MgI2 to moles of MgI2 we do this using the formula weight of MgI2 2781 grams mole in order for our units to cancel we need to conver the mg to g 35 mg 00035 grams. So the correct answer is a. Calculate Percentage Composition of Oxygen by Mass. For a compound with the molecular.
 Source: kentchemistry.com
Source: kentchemistry.com
First we need to get the mass of MgI2 to moles of MgI2 we do this using the formula weight of MgI2 2781 grams mole in order for our units to cancel we need to conver the mg to g 35 mg 00035 grams. Use stoichiometry and dimensional analysis to calculate the number of atoms in 5 moles of NaOH. So in 1 mole of sodium phosphate there will be 3 mole of sodium ions and 1 mole of phosphate ions. Number of ions left textnumber of moles righttextX left N_A right where N_A Avogadros number. The simplest ratio of ions within the structure is 1 calcium ion for every 2 chloride ions giving a formula unit of CaCl 2.
 Source: numerade.com
Source: numerade.com
We also know that 1 mathrmmol of adrenaline weighs 183 mathrmg. The mathematical equation N n N A can also be used to find the number of atoms of each element in a known amount in moles of a compound. How to Find Moles. How to find moles in the solution is to calculate how many molecules the solution contains. Atomic mass of Cu 6355.
 Source: khanacademy.org
Source: khanacademy.org
100 g CaCl 2. Moles of element n moles elementone mole of compound K - 1 mole of K for 1 mole of KNO3. The mathematical equation N n N A can also be used to find the number of atoms of each element in a known amount in moles of a compound. 10 moles of sodium ions 10 6022 10 23 6022 10 24 sodium ions. 2K and SO42- ions.
 Source: slideplayer.com
Source: slideplayer.com
First we need to get the mass of MgI2 to moles of MgI2 we do this using the formula weight of MgI2 2781 grams mole in order for our units to cancel we need to conver the mg to g 35 mg 00035 grams. Atomic mass of CuCl 2 1 6355 2 3545 Atomic mass of CuCl 2 6355 709. Moles of A in 100 grams of the compound. We now know that in every mole of adrenaline there are 48 mathrmg of oxygen. So given 25 moles of CaCl2 when dissolved in a suitable solvent will give you 25 moles of Clacium ions 1x2.
 Source: youtube.com
Source: youtube.com
Number of ions left textnumber of moles righttextX left N_A right where N_A Avogadros number. We now know that in every mole of adrenaline there are 48 mathrmg of oxygen. Use stoichiometry and dimensional analysis to calculate the number of atoms in 5 moles of NaOH. O - 300495 mol 0148 mol. Number of ions left textnumber of moles righttextX left N_A right where N_A Avogadros number.
 Source: youtube.com
Source: youtube.com
Execute I require to obtain the number mole of every ion current from the calculated total variety of moles because that the molecule. Then add the ion count from both compounds to get the total ions. Yes exactly there space three ions present so every you should do is multiply 0599 through three and you will have your last answer. Consequently how many moles of chloride ions are there in 2 moles of calcium chloride. NaClaq Na aq Cl aq So if every mole of sodium chloride produces One mole of sodium cations it follows that the number of moles of sodium cation present in your solution will be equal to the number of moles of sodium chloride you dissolved to create this solution.
 Source: youtube.com
Source: youtube.com
10 moles of water molecules 10 6022 10 23 6022 10 24 water molecules. To acquire the variety of moles divide the mass of compound by the molar mass of the compound expressed in grams. Yes exactly there space three ions present so every you should do is multiply 0599 through three and you will have your last answer. To find the molarity of the ions first determine the molarity of the solute and the ion-to-solute ratio. Each mole of the salt will contain one mole of Calcium and two moles of Chlorine.
 Source: numerade.com
Source: numerade.com
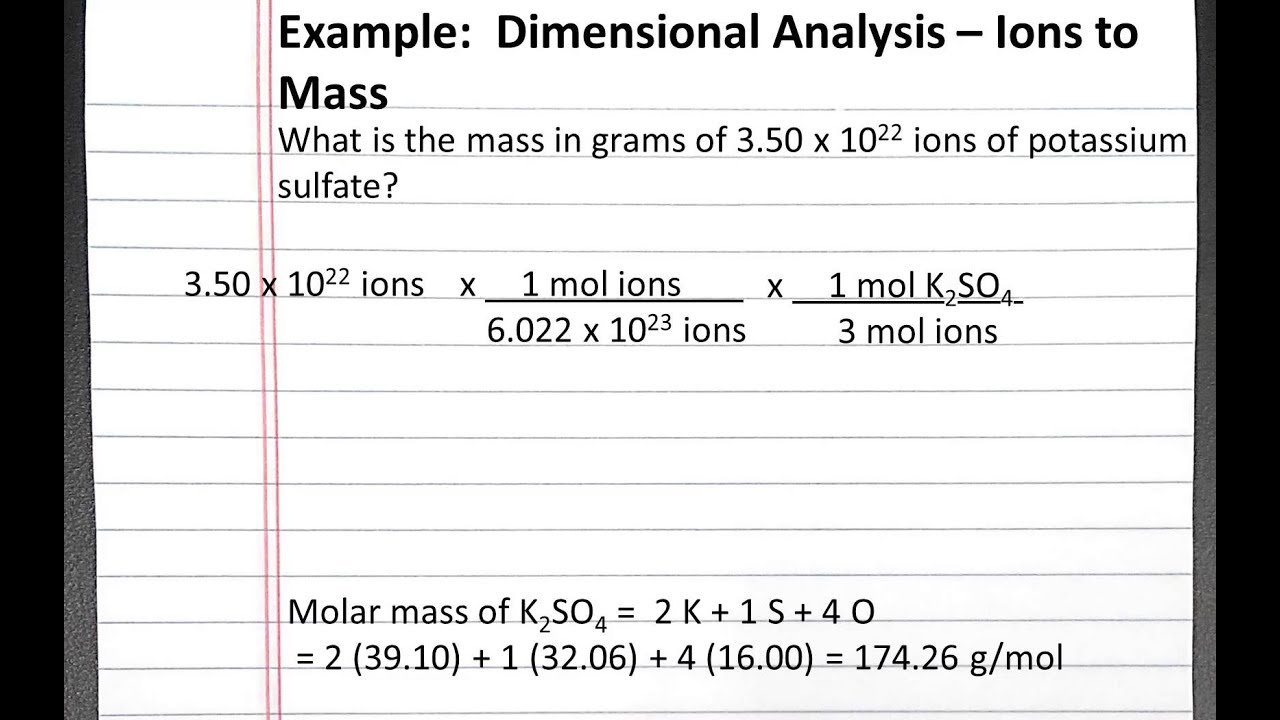
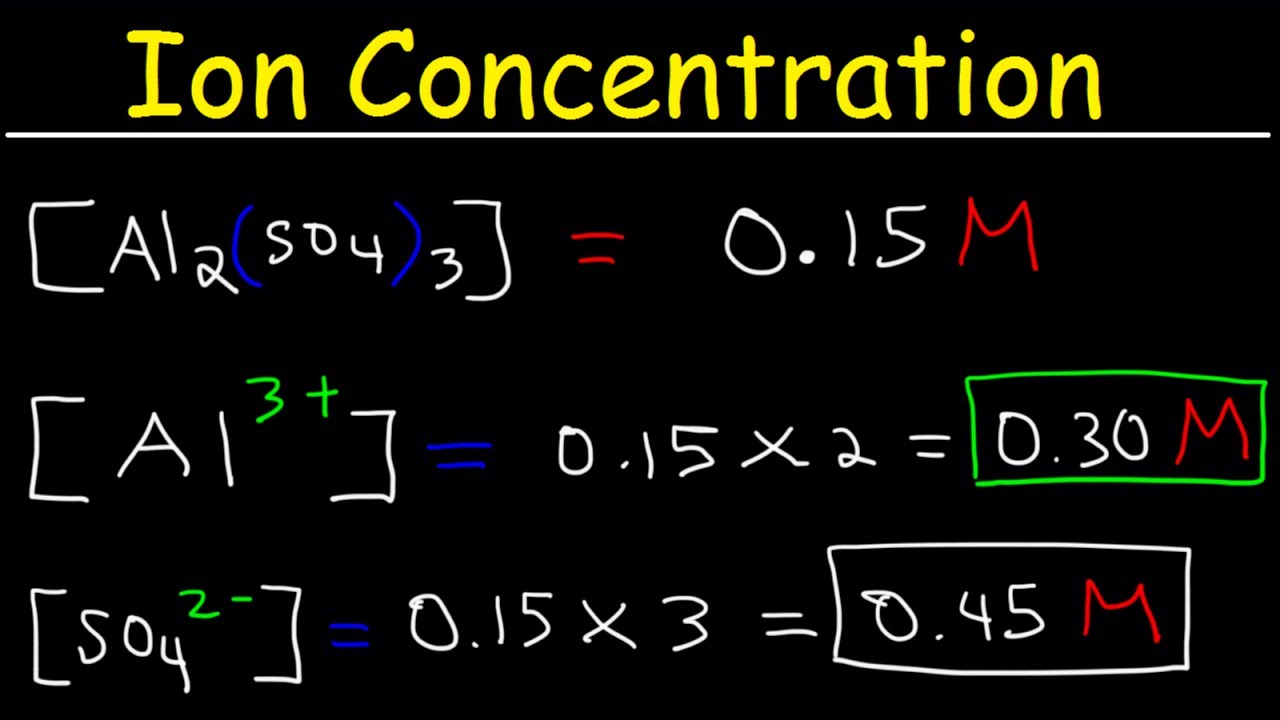
How do you find the number of ions in a formula. 2 moles x 602 x 1023 ionsmole 12 x 1024 ions. If the focus of Na ion on this resolution is 02 molar discover x within the components of compound. Calculate Percentage Composition of Oxygen by Mass. N 1 mole of N for 1 mole of KNO3.
 Source: youtube.com
Source: youtube.com
Then add the ion count from both compounds to get the total ions. O - 300495 mol 0148 mol. If the focus of Na ion on this resolution is 02 molar discover x within the components of compound. Moles of A in 100 grams of the compound. 200 mL H 2 O.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find the moles of ions in a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.