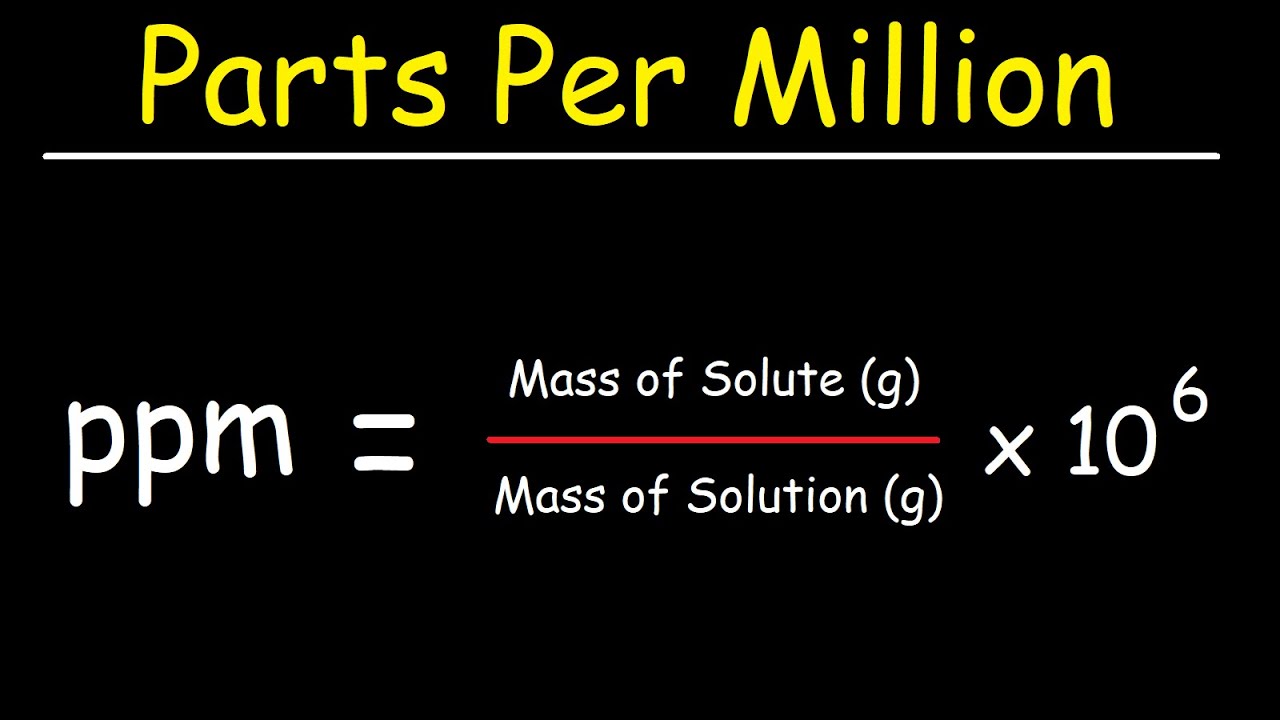Your How to find the mole ratio of a compound images are available. How to find the mole ratio of a compound are a topic that is being searched for and liked by netizens today. You can Find and Download the How to find the mole ratio of a compound files here. Get all royalty-free photos.
If you’re looking for how to find the mole ratio of a compound pictures information connected with to the how to find the mole ratio of a compound keyword, you have pay a visit to the right site. Our website always gives you hints for downloading the highest quality video and image content, please kindly search and locate more enlightening video articles and graphics that fit your interests.
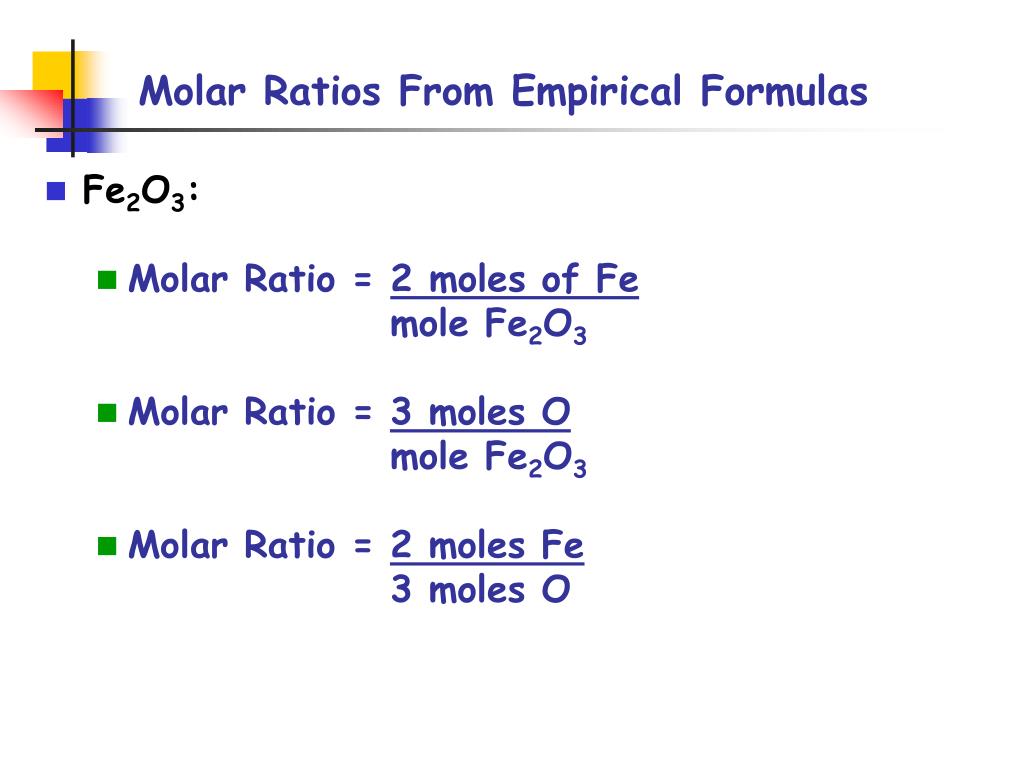
How To Find The Mole Ratio Of A Compound. Use every parts molar mass to transform the grams of every ingredient to moles. The ratio between them is the molar ratio. Feb 09 2021 Steps to find out empirical system. What is the Empirical Formula.
 Chapter 11 Stoichiometry Flashcards Quizlet From quizlet.com
Chapter 11 Stoichiometry Flashcards Quizlet From quizlet.com
Feb 09 2021 Steps to find out empirical system. Moles H 458 g H x 1 mol H 101 g H 453 mol H. Active 6 years 5 months ago. 2 mol O 2. Otherwise multiply the mole ratio by a number which gives two whole numbers for the ratio within 01 range of the whole number. Divide moles of water by the moles of anhydrate to get the mole ratio.
The atomic ratio for this compound is 1C2H1O.
0008800088 is 125. For every 1 mole of O2 used 2 moles of H2O are formed. F is the number of reactive groups per mole of compound. Compound that has a specific number of water molecules bound to its atoms. 2 mol O 2. You can use the mole ratio of elements in a compound to determine the empirical formula of a compound.
 Source: slideserve.com
Source: slideserve.com
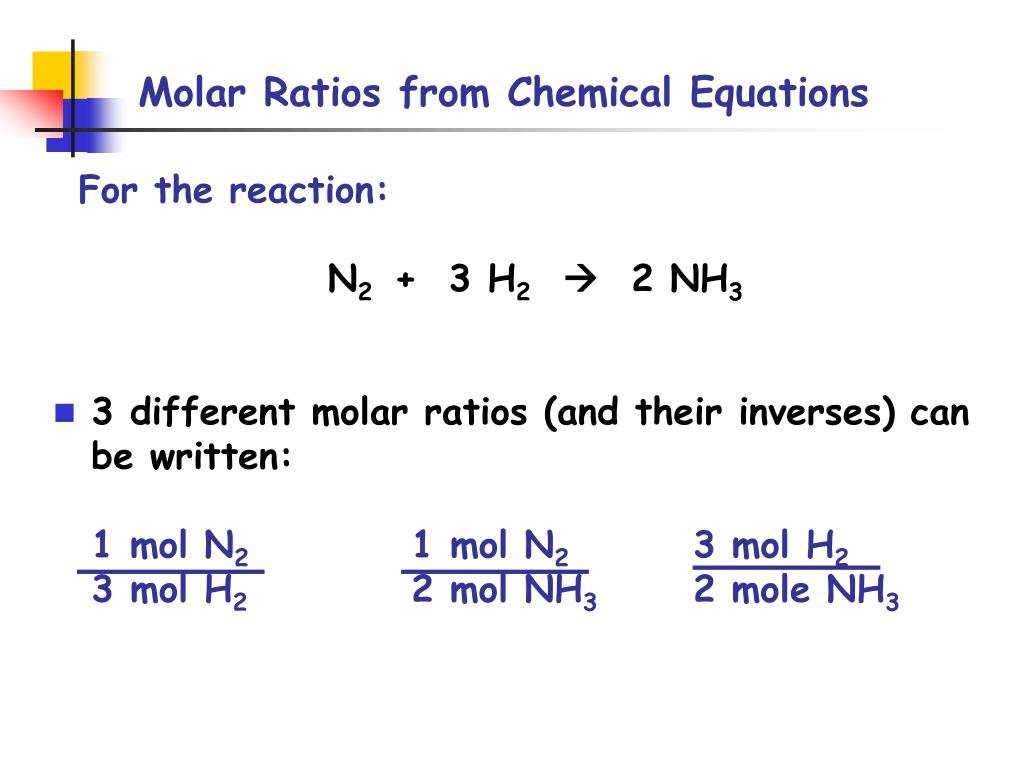
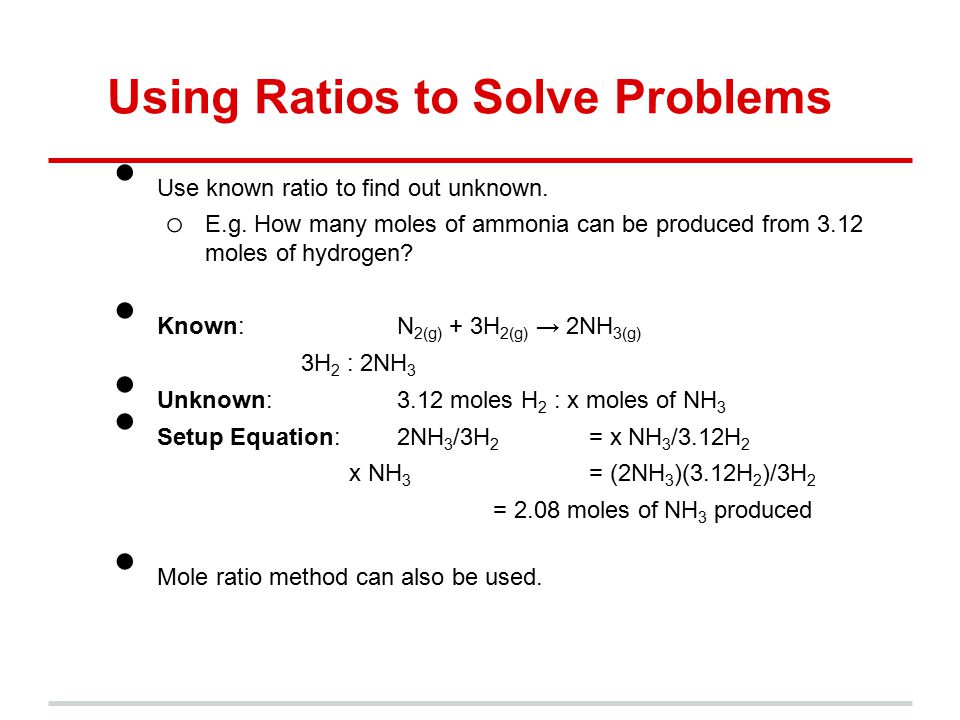
The mole ratio may be determined by examining the coefficients in front of formulas in a balanced chemical equation. Alternatively you can utilize an online mole fraction calculator to determine the mole fraction moles of solute and moles of solvent-based on the input parameters. Find the empirical formula of a compound that has 666 of Carbon C 112 of Hydrogen H 222 of Oxygen O. 120g Al 1 mol Al 2698g Al 0044 48 mol Al. 602 x 1023 atoms Zn1 mol Zn.
 Source: chem.libretexts.org
Source: chem.libretexts.org
What is the Empirical Formula. Round off the mole ratio to the nearest whole number if less than 019 or greater than 081 fractions. 2 mol O 2. 240g I₂ 1 mol I2 2538g I₂ 0009 456 mol I2. 2 mol H 2 O.
 Source: youtube.com
Source: youtube.com
305 not 3051 Show your calculation and round appropriately the ratio you get. Mole ratio of HS mole ratio of SS mole ratio of OS DONE. I am going to separate these compounds by simple and fractional distillation. Percent by mass of each element in a compound. Alternatively you can utilize an online mole fraction calculator to determine the mole fraction moles of solute and moles of solvent-based on the input parameters.
 Source: nagwa.com
Source: nagwa.com
Therefore in 1 mol of this compound there is 1 mole of carbon 2 moles of hydrogen and 1 mole of oxygen. To calculate the mole ratios of the elements you will divide each molar amount by 102 mol. A 25 to 1 is really a 5 to 2 ratio. Please note that using a 33 ratio in a calculation is equivalent to using a 11 ratio. Find the empirical formula of a compound that has 666 of Carbon C 112 of Hydrogen H 222 of Oxygen O.
 Source: nagwa.com
Source: nagwa.com
The numbers of moles of each element are in the same ratio as the number of atoms C H and O in vitamin C. The atomic ratio for this compound is 1C2H1O. Otherwise multiply the mole ratio by a number which gives two whole numbers for the ratio within 01 range of the whole number. Write the chemical formula of the compound with the symbols and. Empirical formula would be Mg 5 O 4.
 Source: youtube.com
Source: youtube.com
Mole ratio of HS mole ratio of SS mole ratio of OS DONE. From the coefficients of the equation the mole ratio is 33. However this reduces to a 11 ratio. 1 or 5 4. The mole ratio may be determined by examining the coefficients in front of formulas in a balanced chemical equation.
 Source: slideplayer.com
Source: slideplayer.com
Find the number of atoms of each element from the Mole Ratio. Active 6 years 5 months ago. Dividing through by 00088 the ratio Mg. 2 mol O 2. Mass in grams of one mole of any pure substance.
 Source: youtube.com
Source: youtube.com
Moles O 545 g O x 1 mol O 1600 g O 341 mol O. Dividing through by 00088 the ratio Mg. Feb 09 2021 Steps to find out empirical system. For every 1 mole of O2 used 2 moles of H2O are formed. Alternatively you can utilize an online mole fraction calculator to determine the mole fraction moles of solute and moles of solvent-based on the input parameters.
 Source: youtube.com
Source: youtube.com
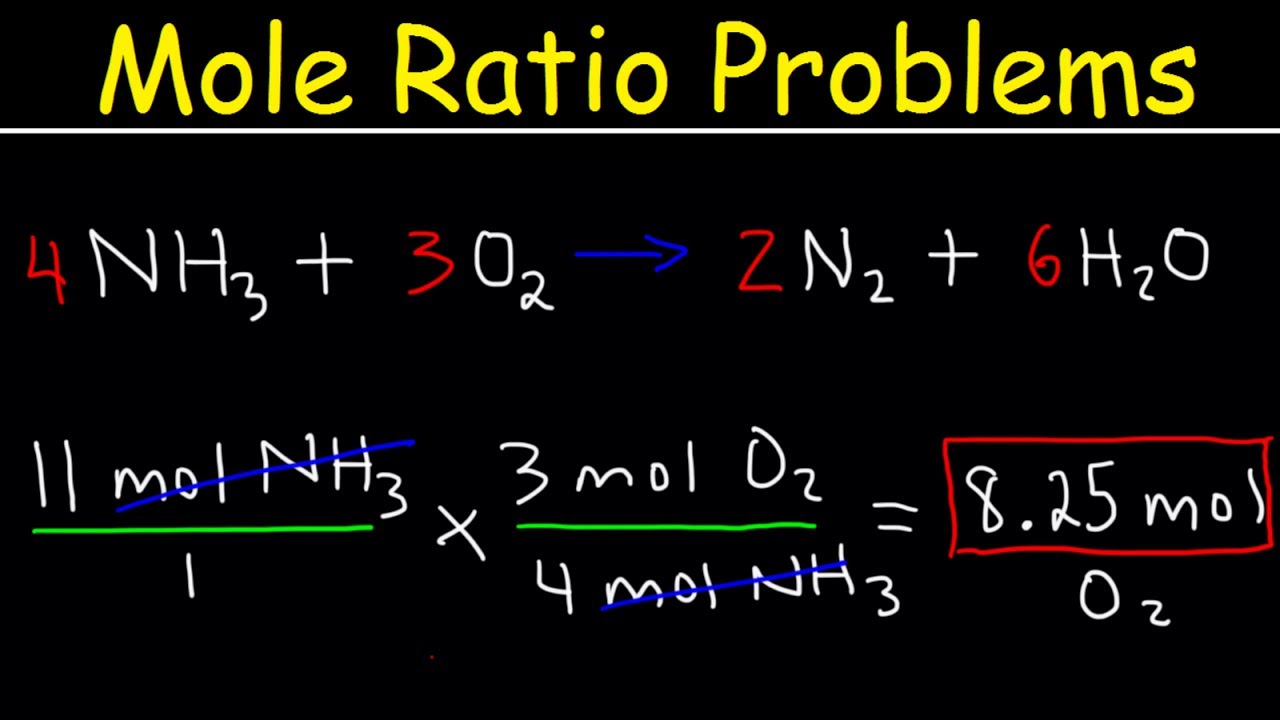
Compound that has a specific number of water molecules bound to its atoms. How to calculate mole ratio of two volatile compounds in distillation. For the reaction2 H2g O2g 2 H2Og The mole ratio between O2 and H2O is 12. Please note that using a 33 ratio in a calculation is equivalent to using a 11 ratio. Choose a signal and calculates the area of H separately of each compound.
 Source: slideplayer.com
Source: slideplayer.com
1 mol CH 4. Calculate the mole ratios of the elements in the compound. The polyol is a trihydroxy compound and therefore has an f of 3 while the diidsocyanate and chain extender have fs of two as each has two. O is 001100088. Get the mass of each element by assuming a certain overall mass for thesample 100 g is a good mass to assume when working with percentages.
 Source: youtube.com
Source: youtube.com
Therefore in 1 mol of this compound there is 1 mole of carbon 2 moles of hydrogen and 1 mole of oxygen. 1 mol CO 2. What is the Empirical Formula. The polyol is a trihydroxy compound and therefore has an f of 3 while the diidsocyanate and chain extender have fs of two as each has two. Feb 09 2021 Steps to find out empirical system.
 Source: slideserve.com
Source: slideserve.com
Ask Question Asked 8 years 2 months ago. O is 001100088. Mass in grams of one mole of any pure substance. In other words 1 mol of methane will produced 1 mole of carbon dioxide as long as the reaction goes to completion and there is plenty of oxygen present. O is 5.
 Source: quizlet.com
Source: quizlet.com
Round off the mole ratio to the nearest whole number if less than 019 or greater than 081 fractions. To calculate the mole ratios of the elements you will divide each molar amount by 102 mol. Please note that using a 33 ratio in a calculation is equivalent to using a 11 ratio. Use every parts molar mass to transform the grams of every ingredient to moles. These molar ratios can also be expressed as fractions.
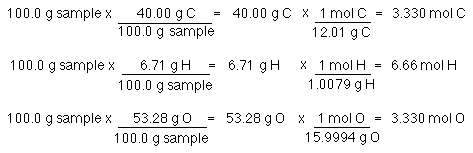 Source: westfield.ma.edu
Source: westfield.ma.edu
1 or 5 4. 240g I₂ 1 mol I2 2538g I₂ 0009 456 mol I2. Round each ratio to the nearest whole number. Viewed 9k times 4 1 begingroup I have two volatile compounds such as ethyl acetate and ethyl propionate. O is 5.
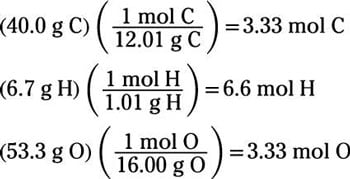 Source: dummies.com
Source: dummies.com
2 mol H 2 O. Mass in grams of one mole of any pure substance. The polyol is a trihydroxy compound and therefore has an f of 3 while the diidsocyanate and chain extender have fs of two as each has two. 0008800088 is 125. Divide by fractional component of each mole value.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find the mole ratio of a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.