Your How to find the mole of an atom images are ready. How to find the mole of an atom are a topic that is being searched for and liked by netizens today. You can Download the How to find the mole of an atom files here. Find and Download all free images.
If you’re looking for how to find the mole of an atom pictures information related to the how to find the mole of an atom interest, you have pay a visit to the right blog. Our site frequently gives you suggestions for viewing the maximum quality video and image content, please kindly hunt and locate more informative video content and graphics that fit your interests.
How To Find The Mole Of An Atom. From moles of a substance one can also find the number of atoms in a sample and vice versa. We can then use the calculated molar mass to convert between mass and number of moles of the. So the number of moles of H atom in it. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl.
 Mole Ratios Mole Ratio Relationship From pinterest.com
Mole Ratios Mole Ratio Relationship From pinterest.com
To Calculate Number of Moles. The representative particles can be atoms molecules or formula units of ionic compounds. Molecular mass of nitrogen N 2 14 x 2 28 g. Avogadros number is a very important relationship to remember. Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. We can then use the calculated molar mass to convert between mass and number of moles of the.
1 mole is equal to 60221415E23 atom.
I asked us to find the number of atoms in each of the following samples so we begin with fourteen point nine five five grams of chromium. Atomicity of calcium Ca molecule is 1. Note that rounding errors may occur so always check the results. For most atoms its around Ryans answer. NaCl Na Cl. The molar mass of a substance is the mass in grams of 1 mole of the substance.
 Source: pinterest.com
Source: pinterest.com
1 mole of a substance contains 6022 x 10 23 molecules. So the number of moles of H atom in it. Use this page to learn how to convert between moles and atoms. Therefore NaCl 584538 gL. Type in your own numbers in the form to convert the units.
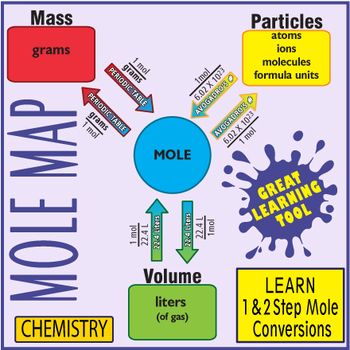 Source: pinterest.com
Source: pinterest.com
Fe is a monoatomic molecule. As shown in this video we can obtain a substances molar mass by summing the molar masses of its component atoms. Use this page to learn how to convert between moles and atoms. Multiply the relative atomic mass by the molar mass constant. In this video well learn to how to determine the number of atoms in one mole of a substance.
 Source: pinterest.com
Source: pinterest.com
From moles of a substance one can also find the number of atoms in a sample and vice versa. Note that rounding errors may occur so always check the results. We can then use the calculated molar mass to convert between mass and number of moles of the. Number of moles mass relative formula mass. 1 mole of a substance contains 6022 x 10 23 molecules.
 Source: pinterest.com
Source: pinterest.com
The bridge between atoms and moles is Avogadros number 602210 23Avogadros number is typically dimensionless but when it defines the mole it can be expressed as 602210 23 elementary entitiesmol. For most atoms its around Ryans answer. The molar mass of any substance is the mass in grams of one mole of representative particles of that substance. To convert moles of atoms divide the atom amount by Avogadros number. From moles of a substance one can also find the number of atoms in a sample and vice versa.
 Source: pinterest.com
Source: pinterest.com
It can also be. Atom percent Mole percent of first element in the binary alloy is calculated from mass percent and the atomic masses of the individual elements and is represented as X 1 100 m 1 A 2 m 1 A 2 100-m 1 A 1 or atom_percent_1 100 Mass percent of the first element Atomic mass of second element Mass percent of the first element Atomic mass of second element. Number of moles mass relative formula mass. As shown in this video we can obtain a substances molar mass by summing the molar masses of its component atoms. So to find the amount of atoms in a mole of NaCl we multiply the two atoms Na and Cl by 602 1024 to give us 1204 1024 atoms in a mol of NaCl.
 Source: pinterest.com
Source: pinterest.com
We can then use the calculated molar mass to convert between mass and number of moles of the. We can then use the calculated molar mass to convert between mass and number of moles of the. It is known as the Avogadro number. The Avogadros number is a very important relationship to remember. Avogadros number is a very important relationship to remember.
 Source: pinterest.com
Source: pinterest.com
Note that rounding errors may occur so always check the results. This is a very large number. Example If you want to find the molar mass of common salt Sodium Chloride- NaCl You add the mass of each element of it. Number of moles of Fe Given mass of Fe Molecular mass of Fe. The Avogadros number is a very important relationship to remember.
 Source: pinterest.com
Source: pinterest.com
1 mole is equal to 60221415E23 atom. Avogadros number is a very important relationship to remember. C o s t p e r a t o m 553 93025 c m 2 2325 c m 2 135 g 2698 g 1 m o l 1 m o l 6022 10 23 a t o m s c o s t p e r a t o m 459 10 25. Therefore NaCl 584538 gL. So to find the amount of atoms in a mole of NaCl we multiply the two atoms Na and Cl by 602 1024 to give us 1204 1024 atoms in a mol of NaCl.
 Source: pinterest.com
Source: pinterest.com
Use this page to learn how to convert between moles and atoms. This is defined as 0001 kilogram per mole or 1 gram per mole. I asked us to find the number of atoms in each of the following samples so we begin with fourteen point nine five five grams of chromium. Calculating molar mass and number of moles. Number of moles of Fe Given mass of Fe Molecular mass of Fe.
 Source: pinterest.com
Source: pinterest.com
So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl. So to find the amount of atoms in a mole of NaCl we multiply the two atoms Na and Cl by 602 1024 to give us 1204 1024 atoms in a mol of NaCl. This can be rearranged to find the mass if the number of moles and molar mass its relative formula mass in grams are known. Mass of one nitrogen atom and nitrogen molecule in kg. To Calculate Number of Moles.
 Source: pinterest.com
Source: pinterest.com
In this video well learn to how to determine the number of atoms in one mole of a substance. Each nitrate ion contains one nitrogen atom and three oxygen atoms. Number of moles mass relative formula mass. I asked us to find the number of atoms in each of the following samples so we begin with fourteen point nine five five grams of chromium. As shown in this video we can obtain a substances molar mass by summing the molar masses of its component atoms.
 Source: nl.pinterest.com
Source: nl.pinterest.com
This can be rearranged to find the mass if the number of moles and molar mass its relative formula mass in grams are known. Avogadros number is a very important relationship to remember. Calculating molar mass and number of moles. Molecular mass of nitrogen N 2 14 x 2 28 g. Now number of moles of CH 4 in 52 1024 molecules is.
 Source: pinterest.com
Source: pinterest.com
One mole of atoms contains 6 x 10 23 atoms no matter what element it is. Molecular mass of nitrogen N 2 14 x 2 28 g. 785 g of Fe at. In this video well learn to how to determine the number of atoms in one mole of a substance. Fe is a monoatomic molecule.
 Source: pinterest.com
Source: pinterest.com
As shown in this video we can obtain a substances molar mass by summing the molar masses of its component atoms. Example If you want to find the molar mass of common salt Sodium Chloride- NaCl You add the mass of each element of it. To find the amount of atoms in a mole we multiply the atoms in an entity by the number above. Each nitrate ion contains one nitrogen atom and three oxygen atoms. Number of moles of Fe.
 Source: pinterest.com
Source: pinterest.com
1 mole is equal to 60221415E23 atom. The atom is the smallest particle of a chemical element that can exist. For most atoms its around Ryans answer. NaCl 229898 gL 354530 gL. Use this page to learn how to convert between moles and atoms.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to find the mole of an atom by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






