Your How to find the mass of moles of a compound images are available in this site. How to find the mass of moles of a compound are a topic that is being searched for and liked by netizens now. You can Download the How to find the mass of moles of a compound files here. Download all royalty-free photos and vectors.
If you’re looking for how to find the mass of moles of a compound images information related to the how to find the mass of moles of a compound interest, you have visit the right site. Our site frequently gives you suggestions for seeing the maximum quality video and image content, please kindly hunt and locate more enlightening video content and graphics that match your interests.
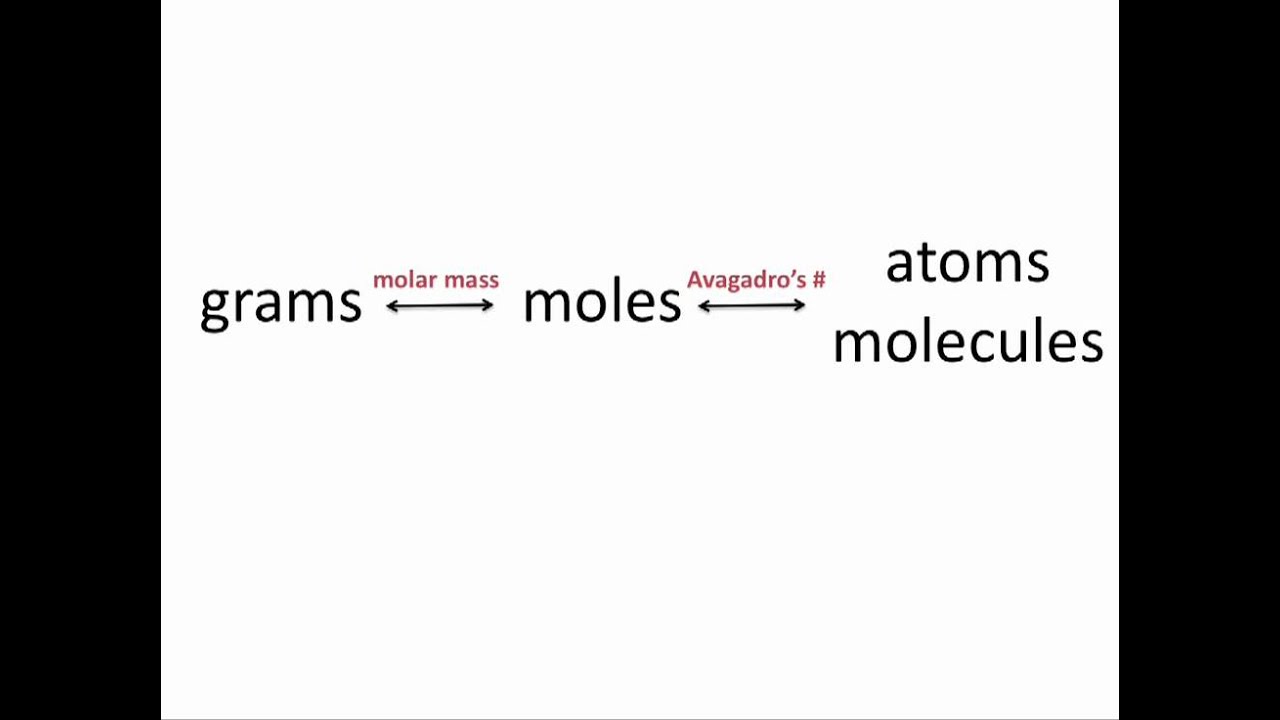
How To Find The Mass Of Moles Of A Compound. To go from grams to moles divide the grams by the molar mass. Ask me questions on Facebook. The units for molar mass are therefore gramsmole. CONVERTING MASS TO MOLES When converting mass to moles follow these steps.
 Calculating Molar Mass And Number Of Moles Worked Example Video Khan Academy From khanacademy.org
Calculating Molar Mass And Number Of Moles Worked Example Video Khan Academy From khanacademy.org
One mole of a compound contains Avogadros number 6022 x 10 23 of molecules molecular compound or formula units ionic compoundThe molar mass of a compound tells you the mass of 1 mole of that substance. Multiply the elements atomic mass by the number of atoms of that element in the compound. In other words it tells you the number of. To find the molar mass of a compound. Molecules or formula units present in a particular chemical sample. Multiply the atomic weight of each element with its number of atoms present in the compound.
The atomic masses go as follows-.
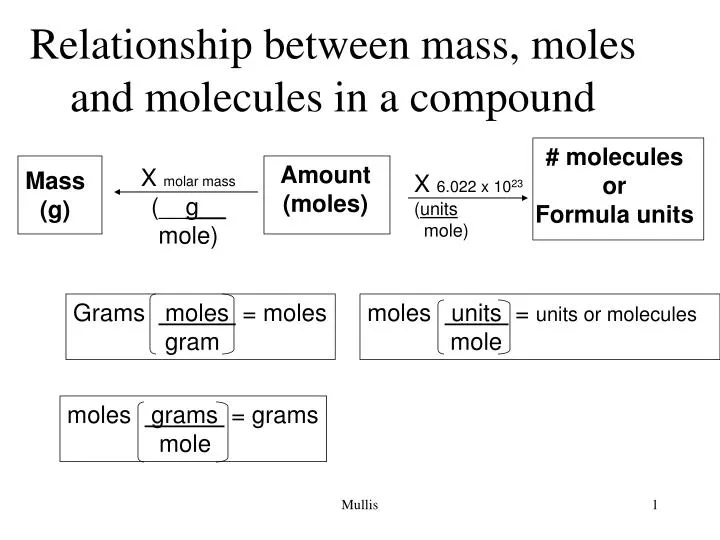
Molecules or formula units present in a particular chemical sample. First we need to calculate the total atomic mass of our compounds copper II oxide CuO and sugar C6H12O6. Craig Beals explains how to convert between moles of a compound and mass using three simple steps. M nM m 15 x 30. To find the molar mass of a compound. Divide the mass of the molecular substance by its molar mass to get moles.
 Source: youtube.com
Source: youtube.com
Multiply the elements atomic mass by the number of atoms of that element in the compound. To convert between grams and moles you would use the substances molar mass. Find the mass of a substance with a molar mass of 30 Kgmol and number of moles of 15 mol. Then multiply times 6022 1023molecules 1mol. To go from grams to moles divide the grams by the molar mass.
 Source: youtube.com
Source: youtube.com
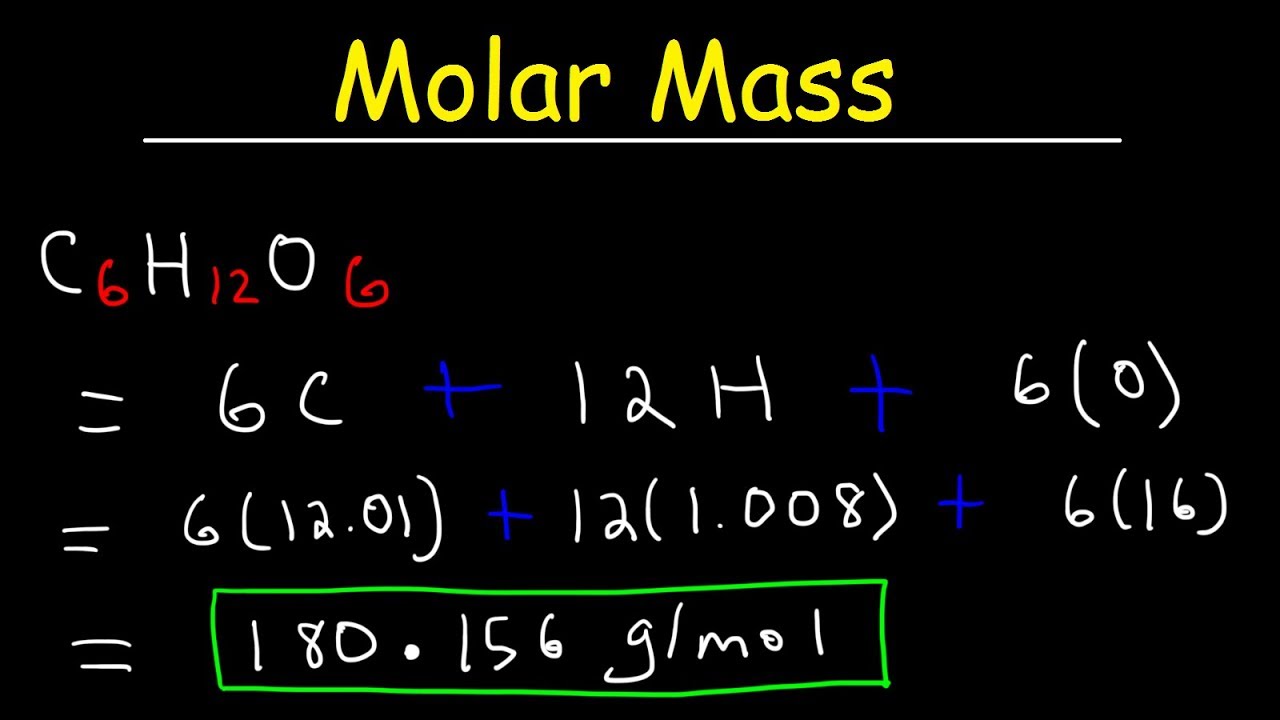
Molecular mass 40078 x 3 3097361 x 2 159994 x 8 molecular mass 120234 6194722 1279952 molecular mass 31017642 from the calculator molecular mass 31018 The final answer uses the correct number of significant figures. To find the molar mass of a compound you have to write the chemical formula list the. First we need to calculate the total atomic mass of our compounds copper II oxide CuO and sugar C6H12O6. To go from grams to moles divide the grams by the molar mass. Find the molar mass of that elementcompound 3.
 Source: youtube.com
Source: youtube.com
The atomic masses go as follows-. Molecular mass 40078 x 3 3097361 x 2 159994 x 8 molecular mass 120234 6194722 1279952 molecular mass 31017642 from the calculator molecular mass 31018 The final answer uses the correct number of significant figures. Add up all and assign unit as gramsmole. Multiply the atomic weight of each element with its number of atoms present in the compound. Ask me questions on Facebook.
 Source: slidetodoc.com
Source: slidetodoc.com
How many moles are in 150 grams of H 2 SO 4. A sample of 12 grams of carbon is equal to one moleThe amount of moles in a substance can be determined using that substances molar mass. To do this you must need to know its chemical formula which is C X 9 H X 13 N O X 3. Use the chemical formula to determine the number of each type of atom present in the compound. Ask me questions on Facebook.
 Source: khanacademy.org
Source: khanacademy.org
Add it all together and put units. A sample of 12 grams of carbon is equal to one moleThe amount of moles in a substance can be determined using that substances molar mass. For finding out this you have to multiply the mass of solute by its molar mass conversion factor. Find the mass of a substance with a molar mass of 30 Kgmol and number of moles of 15 mol. Use the chemical formula to determine the number of each type of atom present in the compound.
 Source: youtube.com
Source: youtube.com
CONVERTING MASS TO MOLES When converting mass to moles follow these steps. For finding out this you have to multiply the mass of solute by its molar mass conversion factor. Multiply the elements atomic mass by the number of atoms of that element in the compound. Divide the mass of the molecular substance by its molar mass to get moles. Multiply the atomic weight of each element with its number of atoms present in the compound.
 Source: slideserve.com
Source: slideserve.com
The mole the unit of measurement for amount of substance is defined in such a way that the molar mass of a compound in gmol is numerically equal to the average mass of one molecule in daltons. M 9 12 g m o l 1 1 13 g m o l 1 14 g m o l 1 3 16 g m o l 1 183 g m o l 1. Thus for example the average mass of a molecule of water is about 180153 daltons and the molar mass of water is about 180153 gmol. Find the mass of a substance with a molar mass of 30 Kgmol and number of moles of 15 mol. Amazingly there are 602x1023 atoms in each of the samples above.
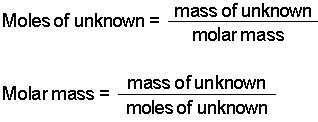 Source: chem.purdue.edu
Source: chem.purdue.edu
The units for molar mass are therefore gramsmole. This chemistry video tutorial explains how to calculate the molar mass of a compound. For finding out this you have to multiply the mass of solute by its molar mass conversion factor. The units for molar mass are therefore gramsmole. Amazingly there are 602x1023 atoms in each of the samples above.
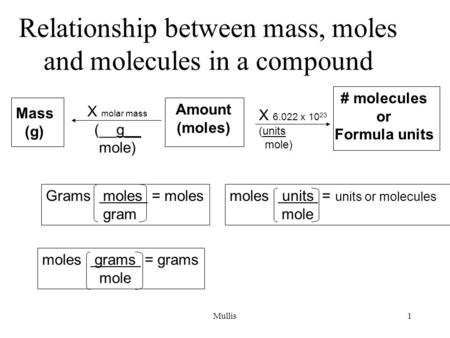 Source: slideplayer.com
Source: slideplayer.com
Multiply the atomic weight from the periodic table of each element by the number of atoms of that element present in the compound. To find the molar mass of a compound. Use dimensional analysis to convert mass to moles 4. Make use of the chemical formula to determine the number of atoms of each element in the compound. M nM m 15 x 30.
 Source: youtube.com
Source: youtube.com
A compound is found to consist of 294 Ca 235 S and 471 O by mass. Add up all and assign unit as gramsmole. Amazingly there are 602x1023 atoms in each of the samples above. To do this you must need to know its chemical formula which is C X 9 H X 13 N O X 3. CONVERTING MASS TO MOLES When converting mass to moles follow these steps.
 Source: youtube.com
Source: youtube.com
Determine what mass you have 2. Now to calculate its molar mass we add up all of the molar masses of each atom. Craig Beals explains how to convert between moles of a compound and mass using three simple steps. Use dimensional analysis to convert mass to moles 4. To find the molar mass of a compound you have to write the chemical formula list the.
 Source: surfguppy.com
Source: surfguppy.com
Add up all and assign unit as gramsmole. Craig Beals explains how to convert between moles of a compound and mass using three simple steps. Then multiply times 6022 1023molecules 1mol. In other words it tells you the number of. Add up all and assign unit as gramsmole.
 Source: youtube.com
Source: youtube.com
M nM m 15 x 30. Find the Molar Mass of Adrenaline. Use the chemical formula to determine the number of each type of atom present in the compound. This will give you the relative amount that each element contributes to the. To find the molar mass of a compound.
 Source: slideplayer.com
Source: slideplayer.com
M 9 12 g m o l 1 1 13 g m o l 1 14 g m o l 1 3 16 g m o l 1 183 g m o l 1. Knowing how many screws of a certain size makes up 1 kg allows you to convert back and. Amazingly there are 602x1023 atoms in each of the samples above. Therefore the units of molar mass are gramsmoleHow to find the molar mass of a compound. Find the mass of a substance with a molar mass of 30 Kgmol and number of moles of 15 mol.
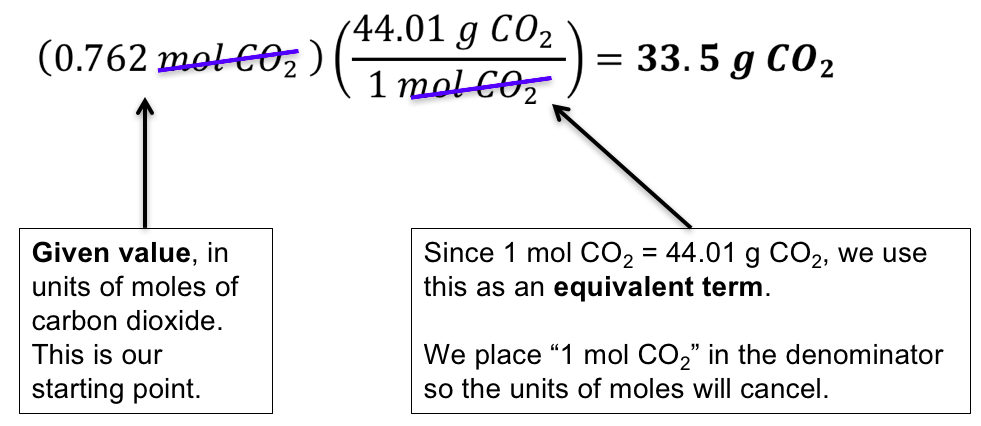 Source: wisc.pb.unizin.org
Source: wisc.pb.unizin.org
To find the molar mass of a compound. Use dimensional analysis to convert mass to moles 4. Multiply the elements atomic mass by the number of atoms of that element in the compound. To convert between grams and moles you would use the substances molar mass. Molecular mass 40078 x 3 3097361 x 2 159994 x 8 molecular mass 120234 6194722 1279952 molecular mass 31017642 from the calculator molecular mass 31018 The final answer uses the correct number of significant figures.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find the mass of moles of a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






