Your How to find out how many moles are in a compound images are ready. How to find out how many moles are in a compound are a topic that is being searched for and liked by netizens now. You can Find and Download the How to find out how many moles are in a compound files here. Download all royalty-free vectors.
If you’re looking for how to find out how many moles are in a compound pictures information related to the how to find out how many moles are in a compound topic, you have come to the right site. Our site always gives you suggestions for seeking the maximum quality video and image content, please kindly hunt and find more enlightening video articles and images that match your interests.
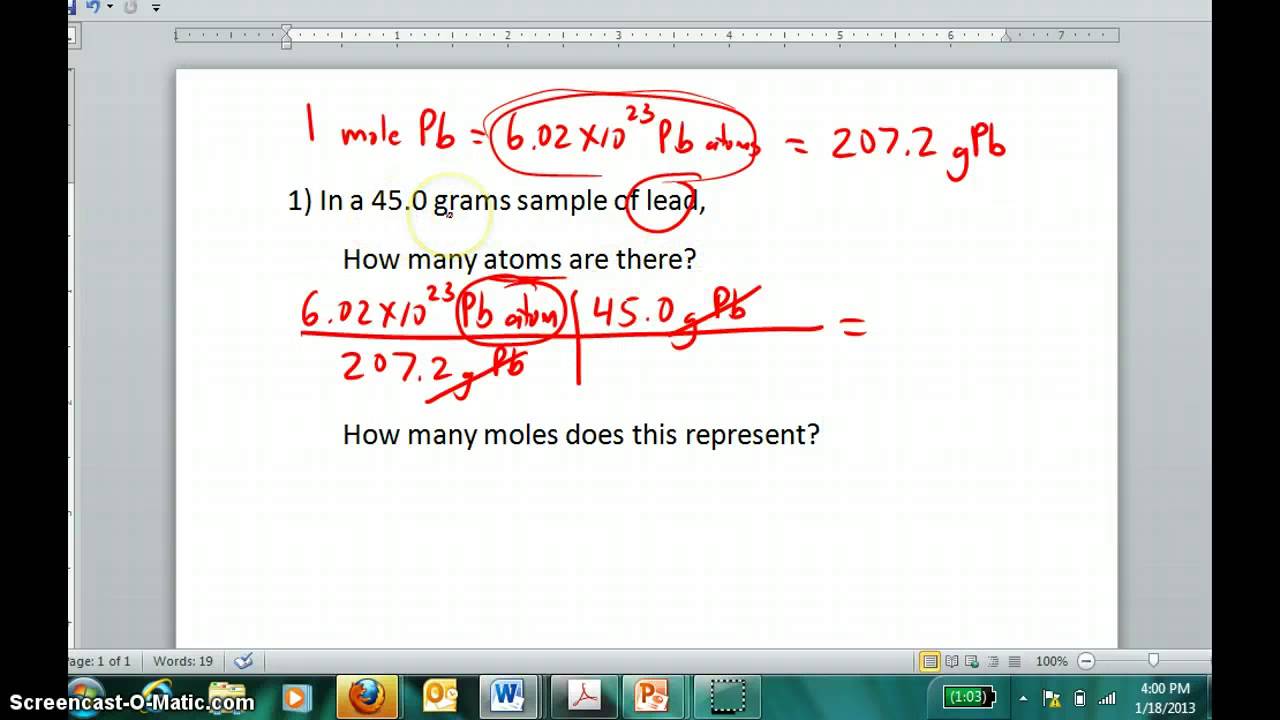
How To Find Out How Many Moles Are In A Compound. Multiply both the values. 25000 g of Cl2 are used and there are 70506 gmol of Cl2. If you have a sample that contains only atoms of a particular element weigh the sample in grams and divide by the atomic weight of the element. Popular Answers 1 Calculate he number of moles you have by taking the Mass molar mass.
 A Mole Ratio Is The Ratio Amounts Of The Entities In A Chemical Reaction Chemistry Worksheets Chemistry Lessons Persuasive Writing Prompts From pinterest.com
A Mole Ratio Is The Ratio Amounts Of The Entities In A Chemical Reaction Chemistry Worksheets Chemistry Lessons Persuasive Writing Prompts From pinterest.com
500 22990 21749. Molarity moles of solute litres of solution. One mole of a compound contains Avogadros number 6022 x 10 23 of molecules molecular compound or formula units ionic compoundThe molar mass of a compound tells you the mass of 1 mole of that substance. To go from molecules to moles divide the numbers of molecules by 602 x 10 23. Determining Weight of a Known Number of Moles of a Compound If you know how many moles you have of a compound you. Now we can use the rearranged equation.
Determining Weight of a Known Number of Moles of a Compound If you know how many moles you have of a compound you.
The number of molecules in a sample is related to moles of compound 1 mole of SO3 will have 602 x 1023 moleculestherefore if you first convert grams t moles then you can convert moles to number of molecules here is the calculation. The number of molecules in a sample is related to moles of compound 1 mole of SO3 will have 602 x 1023 moleculestherefore if you first convert grams t moles then you can convert moles to number of molecules here is the calculation. To go from molecules to moles divide the numbers of molecules by 602 x 10 23. 500 g of Na are used in this reaction and there are 22990 gmol. Moles mol Molarity M x Volume L 05 x 2. 48382 g x 1 mol 8006 g X mole SO3.
 Source: pinterest.com
Source: pinterest.com
It has a mass that is equal to its relative formula mass. The quotient tells you the number of moles. Find the molar mass of a water molecule H₂O. Number of moles formula is. Number of moles 95 8694.
 Source: pinterest.com
Source: pinterest.com
Calculate how many moles are mentioned in the question. Number of moles Mass of substance Mass of one mole. Popular Answers 1 Calculate he number of moles you have by taking the Mass molar mass. A mole of a molecular compound contains 6 x 1023 molecules. Then 1000 g 151001 gmol.
 Source: pinterest.com
Source: pinterest.com
M 9 12 g m o l 1 1 13 g m o l 1 14 g m o l 1 3 16 g m o l 1 183 g m o l 1. M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988 kg. M r of NaOH 23 16 1 40. Mass of one mole MnO2 8694g. I hope this helped without getting too confusing.
 Source: pinterest.com
Source: pinterest.com
I hope this helped without getting too confusing. Moles mol Molarity M x Volume L 05 x 2. Mass g No. Now we can use the rearranged equation. Divide the mass of the compound in grams by the molar mass you just calculated.
 Source: pinterest.com
Source: pinterest.com
Moles mol x Molar Mass gmol 1 x 5844. Number of moles Mass of substance Mass of one mole. Convert the volume of the water to its mass assuming that the density of pure water is 998 kgm³. So a mole of. 48382 g x 1 mol 8006 g X mole SO3.
 Source: pinterest.com
Source: pinterest.com
Number of moles 95 8694. Number of moles formula is. Number of moles of NaOH mass relative formula mass 20 40 05 mol. I hope this helped without getting too confusing. Take a look at the example of how to calculate moles from grams.
 Source: pinterest.com
Source: pinterest.com
Just as with the previous two examples you would use this number 602 1023 Avogadros number to convert from particles atoms or molecules to moles. 500 22990 21749. Number of moles of NaOH mass relative formula mass 20 40 05 mol. If you have a sample that contains only atoms of a particular element weigh the sample in grams and divide by the atomic weight of the element. 48382 g x 1 mol 8006 g X mole SO3.
 Source: pinterest.com
Source: pinterest.com
Number of moles 1092 mol. Moles mol Molarity M x Volume L 05 x 2. If gramsmolar massmoles then first the molar mass of the compound must be found. Divide the number of grams 155 by 110984 and you get the number of moles. Number of moles formula is.
 Source: pinterest.com
Source: pinterest.com
One mole of a compound contains Avogadros number 6022 x 10 23 of molecules molecular compound or formula units ionic compoundThe molar mass of a compound tells you the mass of 1 mole of that substance. M r of NaOH 23 16 1 40. So a mole of. Divide the number of grams of each reactant by the number of grams per mole for that reactant. Convert the volume of the water to its mass assuming that the density of pure water is 998 kgm³.
 Source: pinterest.com
Source: pinterest.com
Convert the volume of the water to its mass assuming that the density of pure water is 998 kgm³. One mole consists of Avogadro number of atoms. 48382 g x 1 mol 8006 g X mole SO3. Multiply both the values. If gramsmolar massmoles then first the molar mass of the compound must be found.
 Source: in.pinterest.com
Source: in.pinterest.com
Divide this number into the weight on hand to find the number of moles. M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988 kg. M 9 12 g m o l 1 1 13 g m o l 1 14 g m o l 1 3 16 g m o l 1 183 g m o l 1. Popular Answers 1 Calculate he number of moles you have by taking the Mass molar mass. Now we can use the rearranged equation.
 Source: in.pinterest.com
Source: in.pinterest.com
21749 moles of Na are used in this reaction. Number of moles Mass of substance Mass of one mole. The answer is the number of moles of that mass of compound. Divide the number of grams 155 by 110984 and you get the number of moles. First of all before you can use this equation you need to know how many moles of solute are there in the solution.
 Source: pinterest.com
Source: pinterest.com
48382 g x 1 mol 8006 g X mole SO3. Number of moles 95 8694. M 9 12 g m o l 1 1 13 g m o l 1 14 g m o l 1 3 16 g m o l 1 183 g m o l 1. 500 22990 21749. X moles x 602 x 1023 SO3 molecules 1mol SO3 Y SO3 molecules.
 Source: pinterest.com
Source: pinterest.com
So a mole of. 300 grams 84 gramsmole 357 moles. Divide the number of grams of each reactant by the number of grams per mole for that reactant. First you must calculate the number of moles in this solution by rearranging the equation. In other words it tells you the number of.
 Source: pinterest.com
Source: pinterest.com
If you have a sample that contains only atoms of a particular element weigh the sample in grams and divide by the atomic weight of the element. Divide the number of grams 155 by 110984 and you get the number of moles. The number of molecules in a sample is related to moles of compound 1 mole of SO3 will have 602 x 1023 moleculestherefore if you first convert grams t moles then you can convert moles to number of molecules here is the calculation. It has a mass that is equal to its relative formula mass. Multiply that by Avogadros number and youll find out how many atoms the sample contains.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find out how many moles are in a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






