Your How to find number of moles using molar mass and volume images are ready in this website. How to find number of moles using molar mass and volume are a topic that is being searched for and liked by netizens today. You can Get the How to find number of moles using molar mass and volume files here. Find and Download all free vectors.
If you’re searching for how to find number of moles using molar mass and volume pictures information related to the how to find number of moles using molar mass and volume interest, you have come to the ideal site. Our site always provides you with suggestions for viewing the maximum quality video and picture content, please kindly hunt and find more enlightening video articles and graphics that match your interests.
How To Find Number Of Moles Using Molar Mass And Volume. 1 Ba x 13733 2 O x 1600 2 H x 101 8 H 2 O x 1802 31551 gmol Always show the unit for molar mass gmol in your final answer. Convert grams to moles. In one mole of a substance there are 6022 x 10 23 particles. Here is a small map that can help you to.
 Determining Molar Mass Of Unknown Using Mol Mol Ratios Youtube From youtube.com
Determining Molar Mass Of Unknown Using Mol Mol Ratios Youtube From youtube.com
To find the molar volume of substance at a particular temperature divide its molecular mass in grams by its density at that temperature. The following diagrams show how to convert between Mass Moles and Gas Volumes. N Number of moles c Molar Concentration V Volume. Lets solve an example. In order to calculate Molar volume of a substance we can divide the molar mass by its density. From you calculate the molar mass to be 1021 gmol.
Mathematically n m M.
N Vc n 31 x 40 n 1240. 100 mL 1082 g1 mL 108 g. A substances molar mass is calculated by multiplying its relative atomic mass by the molar mass constant 1 gmol. Let x 2 the volume of solute. The official symbol for molarity is c concentration but many people use the old symbol M. Where the value of 6022 x 10 23 particles per mole is called the Avogadro constant with the symbol L or N.
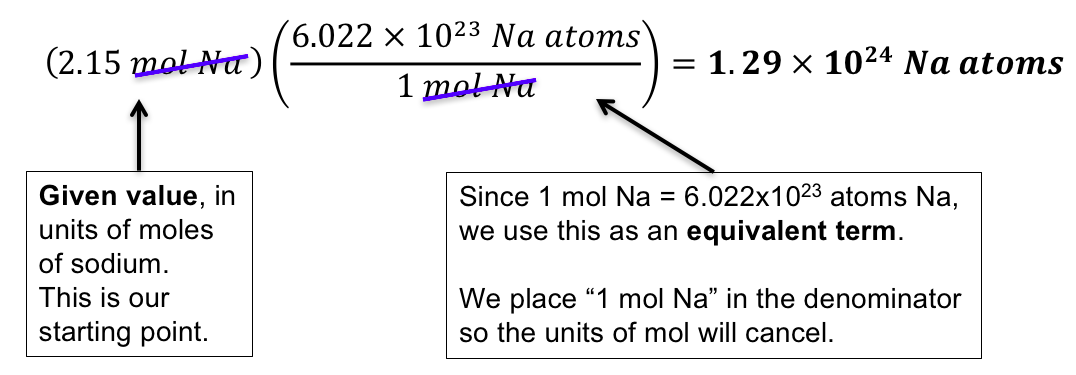 Source: wisc.pb.unizin.org
Source: wisc.pb.unizin.org
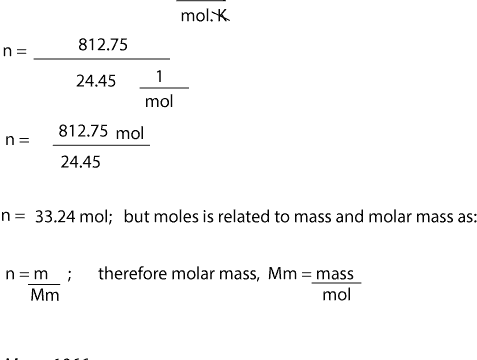
Determine the molar mass from the mass of the unknown and thenumber of moles of unknown. The equation can be rearranged to find the number of. Molar mass sum of atomic masses. 1 Ba x 13733 2 O x 1600 2 H x 101 8 H 2 O x 1802 31551 gmol Always show the unit for molar mass gmol in your final answer. Lets solve an example.
 Source: khanacademy.org
Source: khanacademy.org
Calculate the number of moles of solute. We can rearrange this equation to get the number of moles. Calculating the Number of Moles when Molar Concentration and Volume is Given. Glucose molar mass 180 gmol So can I now find n. 1 Ba x 13733 2 O x 1600 2 H x 101 8 H 2 O x 1802 31551 gmol Always show the unit for molar mass gmol in your final answer.
 Source: slideplayer.com
Source: slideplayer.com
Again we can use proportionalities to determine the amount of solute in some other volume if we know the molarity. To find molarity for our example above simply divide the moles of HCl by the volume in the flask. First of all before you can use this equation you need to know how many moles of solute are there in the solution. In one mole of a substance there are 6022 x 10 23 particles. Determine the molar mass from the mass of the unknown and thenumber of moles of unknown.
 Source: youtube.com
Source: youtube.com
From you calculate the molar mass to be 1021 gmol. Let x 2 the volume of solute. Find the number of moles of a substance with a mass of 22 kg and molar mass of 40 Kgmol. A Volume of CO 2 number of moles of CO 2 224. Volume 05 24 12 dm 3.
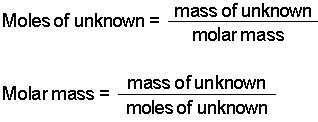 Source: chem.purdue.edu
Source: chem.purdue.edu
Convert millilitres to grams. Let x 2 the volume of solute. A 1 molar abrreviated 1 M is a solution containing 1 mol of solute for every liter of solution. Calculate the volume of solution. Since water has a molecular mass of 180 amu a 100 mole sample of water at 25C would have a volume of 180 g.
 Source: onlinemathlearning.com
Source: onlinemathlearning.com
Determine the moles of unknown the solute from the molarityof the solution and the volume in liters of the solution. The concept of mole in chemical calculations is quite important because it can be a bridge that connects one equation to another. A substances molar mass is calculated by multiplying its relative atomic mass by the molar mass constant 1 gmol. Volume 05 24 12 dm 3. C molar concentration 40 V Volume 31.

Molarity moles of solute litres of solution. Molarity Calculator with Molar Formula As mass volume molarity molar mass then mass. According to the equation the ratio is 13. For finding out this you have to multiply the mass of solute by its molar mass conversion factor. 300mg of calcium chloride contains 00027 moles - or 27 millimoles.
 Source: clutchprep.com
Source: clutchprep.com
Here is a small map that can help you to. In one mole of a substance there are 6022 x 10 23 particles. M Mass 22 M Molar Mass 40. How to find the molar volume of a gas using the ideal gas law. Using Molarity to Find Solution Volume.
 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
Convert millilitres to grams. N m M. 100 mL 1082 g1 mL 108 g. Calculate the number of moles of solute. You have to work out the molar mass of CHO.
 Source: pinterest.com
Source: pinterest.com
Where the value of 6022 x 10 23 particles per mole is called the Avogadro constant with the symbol L or N. Glucose molar mass 180 gmol So can I now find n. You need the concentration of the solution to do this. Calculate the molar mass of the solute. Calculate the number of moles of solute.
 Source: slideplayer.com
Source: slideplayer.com
Lets solve an example. In one mole of a substance there are 6022 x 10 23 particles. 300mg of calcium chloride contains 00027 moles - or 27 millimoles. A Volume of CO 2 number of moles of CO 2 224. Mathematically n m M.
 Source: youtube.com
Source: youtube.com
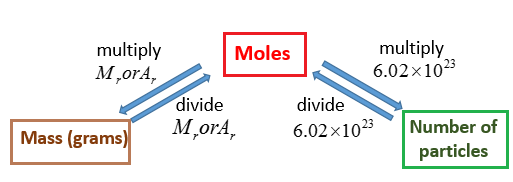
Calculating the Number of Moles when Molar Concentration and Volume is Given. By multiplying a given mass by the molar mass the amount of moles of. Where 12gm is the molar mass of 12C or in other words the mass of 1 mole or 6022 x 1023 atoms of 12C isotope of Carbon. N Number of moles m Mass M Molar Mass. N m M.
 Source: youtube.com
Source: youtube.com
We can rearrange this equation to get the number of moles. Remember that 1 dm 3 1 000 cm 3 so the volume is also 12 000 cm 3. N Vc n 31 x 40 n 1240. V in liters 480cm31000cm3L 048L. Calculate the number of moles of solute.
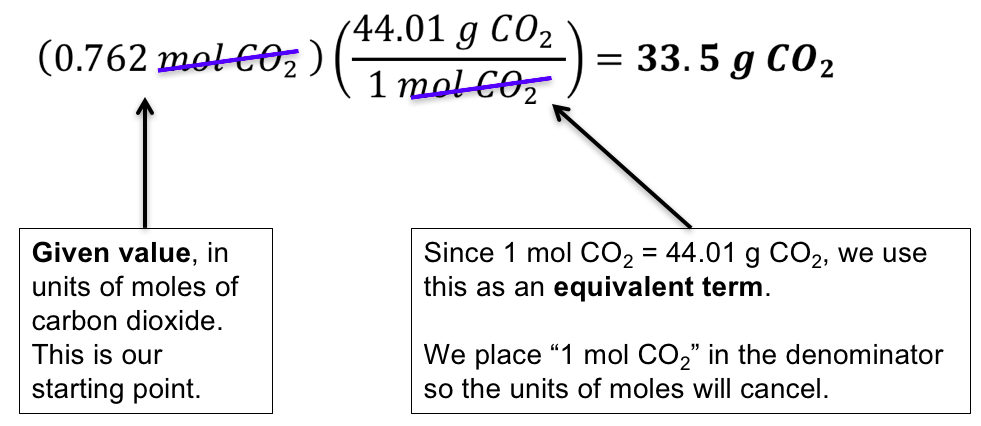 Source: wisc.pb.unizin.org
Source: wisc.pb.unizin.org
C molar concentration 40 V Volume 31. N M V. By multiplying a given mass by the molar mass the amount of moles of. Mathematically n m M. How to find the molar volume of a gas using the ideal gas law.
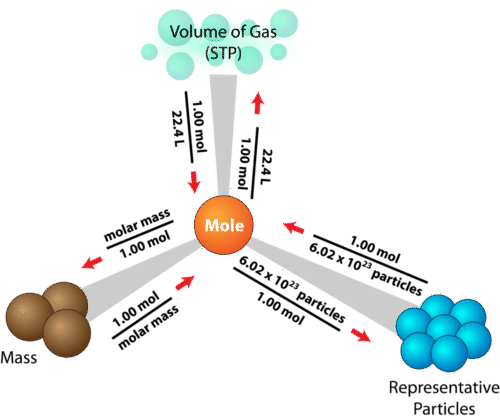
Determine the molar concentration of the unknown in the solutionfrom the observed osmotic pressure. Again we can use proportionalities to determine the amount of solute in some other volume if we know the molarity. Here is a small map that can help you to. Volume 05 24 12 dm 3. Calculate the volume of carbon dioxide gas CO 2 occupied by a 5 moles and b 05 moles of the gas occupied at STP.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find number of moles using molar mass and volume by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






