Your How to find number of moles of each element in a compound images are ready in this website. How to find number of moles of each element in a compound are a topic that is being searched for and liked by netizens today. You can Get the How to find number of moles of each element in a compound files here. Download all royalty-free photos and vectors.
If you’re looking for how to find number of moles of each element in a compound images information linked to the how to find number of moles of each element in a compound keyword, you have pay a visit to the right blog. Our site always provides you with suggestions for seeking the maximum quality video and picture content, please kindly surf and locate more informative video content and graphics that fit your interests.
How To Find Number Of Moles Of Each Element In A Compound. Number of moles Mass of substance Mass of one mole. Note that rounding errors may occur so always check the results. Answer 1 of 3. 5 moles H X 2 S O X 4.
 Moles To Atoms Conversion Chemistry Youtube From youtube.com
Moles To Atoms Conversion Chemistry Youtube From youtube.com
This is Mass Moles. In this manner how do you find the number of moles of an element in a compound. Number of moles 1092 mol. How many moles of each element are present in two moles of the compound. NH 4 2SO 4 2. N the number of moles of a compound.
Now to calculate its molar mass we add up all of the molar masses of each atom.
Number of moles 1092 mol. Mass of one mole MnO2 8694g. To do this you must need to know its chemical formula which is C X 9 H X 13 N O X 3. K - 00495 mol. So The number of moles of each element. Now the only confusing thing for me is the 5 moles.
 Source: youtube.com
Source: youtube.com
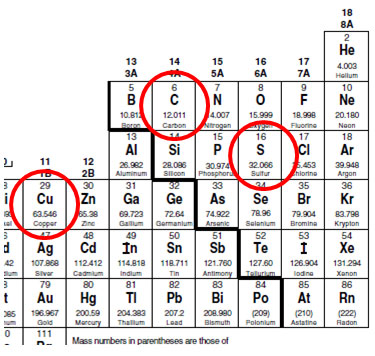
Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. Then 1000 g 151001 gmol X g moles. Now to calculate its molar mass we add up all of the molar masses of each atom. How many moles of each element are present in two moles of the compound. Example If you want to find the molar mass of common salt Sodium Chloride- NaCl You add the mass of each element of it.
 Source: texasgateway.org
Source: texasgateway.org
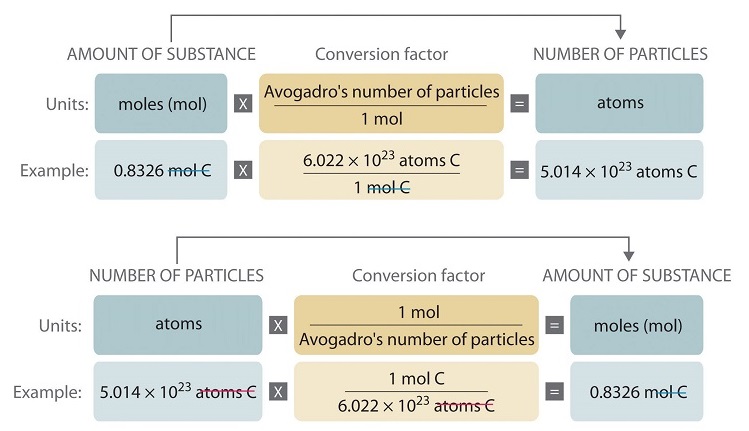
Calculate he number of moles you have by taking the Mass molar mass. N the number of moles of a compound. This is Mass Moles. When you go to the unit from moles multiply by Avogadros number. How do you find the number of atoms of an element in a compound.
 Source: youtube.com
Source: youtube.com
This is Moles Atoms. 10 moles H X 2 602 10 23 602 10 23 atoms. Whenever you go to the mole divide by Avogadros number. For example lets take H X 2 element. N the number of moles of a compound.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Once you have moles multiply by Avogadros number to calculate the number of atoms. 02729 mol C2H6O x 60221023 1 mol 16431022 molecules. Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. You can find the moles of any mass of any compound. 602 x 1023 of them.
 Source: youtube.com
Source: youtube.com
Calculate the number of moles of each component element in the compound moles H moles S moles O DONE Get the answers you need now. Number of moles formula is. 10 moles H X 2 602 10 23 602 10 23 atoms. Divide mass of each by its molar mass gives 00341 mol C 0046 mol H and 00341 mol O. For example lets take H X 2 element.
 Source: slideplayer.com
Source: slideplayer.com
N 1 mole of N for 1 mole of KNO3. Multiply the number of moles by Avogadros number to. 02729 mol C2H6O x 60221023 1 mol 16431022 molecules. Just as with the previous two examples you would use this number 602 1023 Avogadros number to convert from particles atoms or molecules to moles. One mole equals to a very large number of particles.
 Source: study.com
Source: study.com
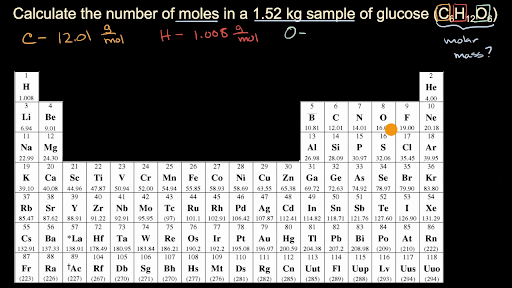
Calculate mass of O by difference. Jeremiah Daniels on How To Find Moles Of An Element In A Compound Given Mass Mar 1 2019 Simply divide the mass with units of grams by the molar mass with units of grams PER mole And so if got a mass of we divide thru by the molar mass. Calculate mass of O by difference. Determining Number of Moles of a Compound With Known Mass Once youve found the molecular weight you know the weight of one mole of a compound. M 9 12 g m o l 1 1 13 g m o l 1 14 g m o l 1 3 16 g m o l 1 183 g m o l 1.
 Source: pinterest.com
Source: pinterest.com
Determining Number of Moles of a Compound With Known Mass Once youve found the molecular weight you know the weight of one mole of a compound. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl. So The number of moles of each element. How many atoms of each element are present in one molecule of the compound. M 9 12 g m o l 1 1 13 g m o l 1 14 g m o l 1 3 16 g m o l 1 183 g m o l 1.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Therefore NaCl 584538 gL. You can find the moles of any mass of any compound. Just as with the previous two examples you would use this number 602 1023 Avogadros number to convert from particles atoms or molecules to moles. In this manner how do you find the number of moles of an element in a compound. Example If we are told the mass of each element present in a compound we can find the formula.
 Source: khanacademy.org
Source: khanacademy.org
Therefore NaCl 584538 gL. So nNNa – n Na N number of atoms 2 1grmol H 2 moles H x 5 moles in the compound 10 moles. You can find the moles of any mass of any compound. 1257g C2H6O x 1 mol C2H6O 46068g 002729 mol C2H6O. 602 x 1023 of them.
Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. Mass of one mole MnO2 8694g. N 1 mole of N for 1 mole of KNO3. O - 300495 mol 0148 mol. In this manner how do you find the number of moles of an element in a compound.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
5 moles H X 2 S O X 4. 602 x 1023 of them. 1257g C2H6O x 1 mol C2H6O 46068g 002729 mol C2H6O. So nNNa – n Na N number of atoms 2 1grmol H 2 moles H x 5 moles in the compound 10 moles. To find the number of moles in a sample simply weigh it and divide the weight by the molecular weight.
 Source: slideserve.com
Source: slideserve.com
1257g C2H6O x 1 mol C2H6O 46068g 002729 mol C2H6O. Write the appropriate chemical formulas for compounds with the following ratios of elements. Therefore NaCl 584538 gL. This is Mass Moles. Second find how many molecules of each element are in it.
 Source: khanacademy.org
Source: khanacademy.org
This is Moles Atoms. Therefore NaCl 584538 gL. If you know the number of moles of. Whereas units such as grams or pounds describe the mass of a chemical moles describe the number of particles – either atoms or molecules – of that compound. Third find of positive charge atoms.
 Source: slideplayer.com
Source: slideplayer.com
Calculate mass of O by difference. N - 00495 mol. Jeremiah Daniels on How To Find Moles Of An Element In A Compound Given Mass Mar 1 2019 Simply divide the mass with units of grams by the molar mass with units of grams PER mole And so if got a mass of we divide thru by the molar mass. To find the number of moles in a sample simply weigh it and divide the weight by the molecular weight. So nNNa – n Na N number of atoms 2 1grmol H 2 moles H x 5 moles in the compound 10 moles.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to find number of moles of each element in a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






