Your How to find number of moles of a compound images are available in this site. How to find number of moles of a compound are a topic that is being searched for and liked by netizens now. You can Download the How to find number of moles of a compound files here. Get all free vectors.
If you’re looking for how to find number of moles of a compound images information related to the how to find number of moles of a compound topic, you have pay a visit to the right blog. Our website always gives you suggestions for downloading the highest quality video and image content, please kindly hunt and locate more informative video articles and images that fit your interests.
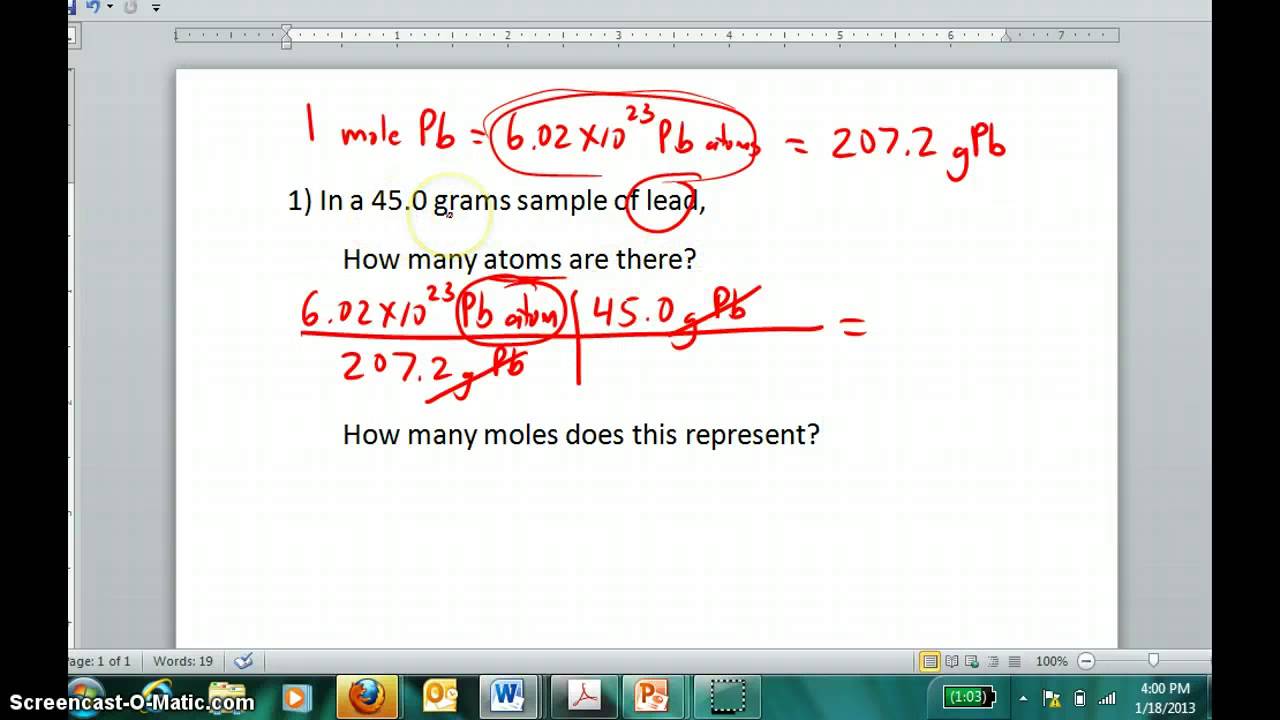
How To Find Number Of Moles Of A Compound. Given mass of compound 158 gm And molar mass of compound 1. The answer is the number of moles of that mass of compound. Moles mol x Molar Mass gmol 1 x 5844. Moles O 212 g O x 1 mol O 1600 g O 133 mol O.
 How To Convert Grams Moles Atoms And Liters In Chemistry In 2021 School Organization Notes School Study Tips Chemistry Quotes From pinterest.com
How To Convert Grams Moles Atoms And Liters In Chemistry In 2021 School Organization Notes School Study Tips Chemistry Quotes From pinterest.com
The mole represents a quantity of substance but relates to the number of atoms or molecules rather than mass or volume. To compute for the molar mass two essential parameters are needed and these parameters are mass m and number of moles n. 25000 70506 035458. Then 1000 g 151001 gmol X g moles. You should always include the units in your calculations. This is the molar mass of the compound.
It has units of grams per mole.
To find the simplest whole number ratio divide each number by the smallest number of moles. Number of moles 95 8694. You have to work out the molar mass of CHO. In other words it tells you the number of. First you must calculate the number of moles in this solution by rearranging the equation. It has units of grams per mole.
 Source: pinterest.com
Source: pinterest.com
Notice how in each step we set up the conversion factor so that the units cancel to give the correct units for the answer. For NaCl the molar mass is 5844 gmol. Calculate he number of moles you have by taking the Mass molar mass. Determine the number of moles in 95g of MnO 2. You have to work out the molar mass of CHO.
 Source: pinterest.com
Source: pinterest.com
Moles mol Molarity M x Volume L 05 x 2. Find out the molar mass of the substance hint. Calculate the concentration of the acid. Numerically this would be 2 1008 1 1600 18016. Moles mol x Molar Mass gmol 1 x 5844.
 Source: pinterest.com
Source: pinterest.com
You calculate the number of moles by dividing the mass of substance by the substances atomic or molecular weight. You can use Molar mass of the substance alone to calculate molar mass. Specifically 1 mole represents 6022 x 1023 atoms or molecules of substance. Just as with the previous two examples you would use this number 602 1023 Avogadros number to convert from particles atoms or molecules to moles. For finding out this you have to multiply the mass of solute by its molar mass conversion factor.
 Source: pinterest.com
Source: pinterest.com
500 g of Na are used in this reaction and there are 22990 gmol. From you calculate the molar mass to be 1021 gmol. Solved Examples On Number Of Moles Formulas. The numbers of moles of each element are in the same ratio as the number of atoms Sn and O in cassiterite. To compute for the molar mass two essential parameters are needed and these parameters are mass m and number of moles n.
 Source: pinterest.com
Source: pinterest.com
Whenever you go to the mole divide by Avogadros number. The molar mass of KClO3 is 122548 gmol. Molarity moles of solute litres of solution. This can be rearranged to find the mass if the number of moles and molar mass its relative formula mass in grams are known. Moles mol x Molar Mass gmol 1 x 5844.
 Source: pinterest.com
Source: pinterest.com
Number of moles formula is. Given mass of compound 158 gm And molar mass of compound 1. Just as with the previous two examples you would use this number 602 1023 Avogadros number to convert from particles atoms or molecules to moles. Moles of NaOH concentration volume. Moles mol Molarity M x Volume L 05 x 2.
 Source: youtube.com
Source: youtube.com
Notice how in each step we set up the conversion factor so that the units cancel to give the correct units for the answer. Now we can use the rearranged equation. Number of moles Mass of substance Mass of one mole. Experts are tested by Chegg as specialists in their subject area. Moles of NaOH concentration volume.
 Source: surfguppy.com
Source: surfguppy.com
Just as with the previous two examples you would use this number 602 1023 Avogadros number to convert from particles atoms or molecules to moles. M Molar mass. You can use Molar mass of the substance alone to calculate molar mass. 500 22990 21749. Divide the mass of the compound in grams by the molar mass you just calculated.
 Source: pinterest.com
Source: pinterest.com
From you calculate the molar mass to be 1021 gmol. The formula for calculating the molar mass. Then 1000 g 151001 gmol X g moles. For example 25 grams of water equals 2518016 or 139 moles. You should always include the units in your calculations.
 Source: pinterest.com
Source: pinterest.com
You calculate the number of moles by dividing the mass of substance by the substances atomic or molecular weight. Divide the number of grams of each reactant by the number of grams per mole for that reactant. Moles of molecules n if you know the number of molecules present N The table below shows the calculation of moles n given then number of particles N. M Molar mass. Calculate the concentration of the acid.
 Source: pinterest.com
Source: pinterest.com
Moles of NaOH concentration volume. The answer is the number of. From you calculate the molar mass to be 1021 gmol. Whenever you go to the mole divide by Avogadros number. Number of moles mass relative formula mass.
 Source: pinterest.com
Source: pinterest.com
Determine the number of moles in 95g of MnO 2. Who are the experts. Specifically 1 mole represents 6022 x 1023 atoms or molecules of substance. This is the molar mass of the compound. Divide the mass of the compound in grams by the molar mass you just calculated.
 Source: pinterest.com
Source: pinterest.com
You should always include the units in your calculations. For finding out this you have to multiply the mass of solute by its molar mass conversion factor. 500 g of Na are used in this reaction and there are 22990 gmol. 500 22990 21749. You should always include the units in your calculations.
 Source: khanacademy.org
Source: khanacademy.org
You should always include the units in your calculations. The answer is the number of. 25000 g of Cl2 are used and there are 70506 gmol of Cl2. Moles O 212 g O x 1 mol O 1600 g O 133 mol O. Divide the number of grams of each reactant by the number of grams per mole for that reactant.
 Source: pinterest.com
Source: pinterest.com
Find out the molar mass of the substance hint. Given mass of compound 158 gm And molar mass of compound 1. If you have 1000 grams. The number of molecules in a sample is related to moles of compound 1 mole of SO3 will have 602 x 1023 moleculestherefore if you first convert grams t moles then you can convert moles to number of molecules here is the calculation. 500 g of Na are used in this reaction and there are 22990 gmol.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find number of moles of a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






