Your How to find moles of unknown compound images are available in this site. How to find moles of unknown compound are a topic that is being searched for and liked by netizens today. You can Get the How to find moles of unknown compound files here. Get all free images.
If you’re searching for how to find moles of unknown compound pictures information related to the how to find moles of unknown compound keyword, you have pay a visit to the ideal site. Our website frequently gives you suggestions for seeking the highest quality video and image content, please kindly search and locate more enlightening video articles and images that fit your interests.
How To Find Moles Of Unknown Compound. M M m M n M 19685 g m o l 1. Then use the molality equation to calculate the moles of solute. How do I find the number of moles of an ion in a compound with an unknown cation. Determine the moles of unknown the solute from the molality of the solution and the mass of solvent in kilograms used to make the solution.
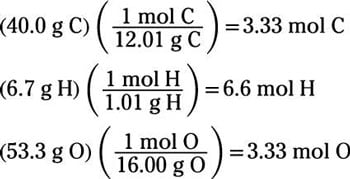 How To Calculate The Empirical Formula Of A Compound Dummies From dummies.com
How To Calculate The Empirical Formula Of A Compound Dummies From dummies.com
Molal moles of solutekg of solvent moles of solute in 1 kg of water in the above example. Molar mass solute. Now click the button calculate molar mass to get the result step 3. First black basketball player in nba. N M n S O X 4. And the product Ca3.
List the known quantities and plan the problem.
Calculate the molality using the change in boiling point or freezing point and the elevation or depression constant. List the known quantities and plan the problem. List the known quantities and plan the problem. To calculate the molality of the solution. Molar mass mass of compound gmoles of compound mol from osmotic pressure. Peach smoothie bowl without yogurt.
 Source: youtube.com
Source: youtube.com
We can now calculate the molar mass of the metal using the information above. Assuming the unknown is soluble in water dissolve 100 grams of the unknown in 1000 ml of water 1 kg of water. Then use the molality equation to calculate the moles of solute. How many moles of carbon hydrogen and. And the product Ca3.
 Source: pinterest.com
Source: pinterest.com
Find the moles of solute from molality by multiplying by the kg of solvent. Example If you want to find the molar mass of common salt Sodium Chloride- NaCl You add the mass of each element of it. Balance the equation in step 1 to a molecular reaction by figuring out what numbers need. What is a tefl qualification equivalent to. List the known quantities and plan the problem.
 Source: khanacademy.org
Source: khanacademy.org
Then divide the grams of solute by the moles to determine the molar mass. Calculate the molar mass of the solute. Mass H 2 O 218 g 0218 kg. Molar mass mass of compound gmoles of compound mol from osmotic pressure. Molality m x kg of solvent moles of solute.
 Source: youtube.com
Source: youtube.com
Using data given to us we first converted grams of released C O X 2 to moles. And the product Ca3. If the sum of percentages of all these elements is not 100 then the difference gives the percentage of oxygen. M M m M n M 19685 g m o l 1. Determine the percentage of each element present in the compound from the mass of each element present in a certain known mass of the compound.
 Source: pinterest.com
Source: pinterest.com
Molar mass solute. M M m M n M 19685 g m o l 1. Determine the moles of unknown the solute from the molality of the solution and the mass of solvent in kilograms used to make the solution. We can now calculate the molar mass of the metal using the information above. Then divide the grams of solute by the moles to determine the molar mass.
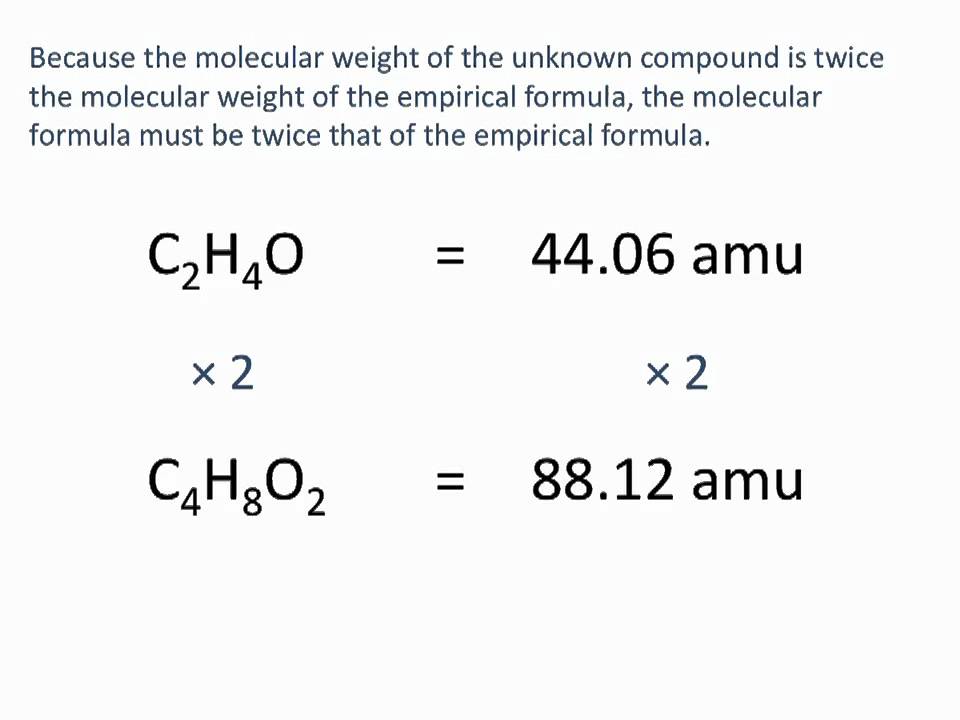 Source: pinterest.com
Source: pinterest.com
M M m M n M 19685 g m o l 1. H C l M X 2 C O X 3 M C l C O X 2 H X 2 O. Then use the molality equation to calculate the moles of solute. List the known quantities and plan the problem. If the sum of percentages of all these elements is not 100 then the difference gives the percentage of oxygen.
 Source: youtube.com
Source: youtube.com
N M n S O X 4. If the sum of percentages of all these elements is not 100 then the difference gives the percentage of oxygen. A 100 g sample of an unknown compound composed of carbon hydrogen and oxygen contains 313 oxygen and eq59 times 1023 eq atoms of hydrogen. N S O X 4 m S O X 4 M S O X 4 635 m m o l. Assuming that the change in boiling point is 1 degree C the result becomes.
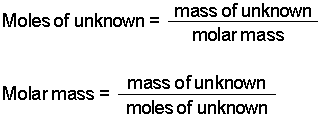 Source: chem.purdue.edu
Source: chem.purdue.edu
Mass solute 387 g. Determine the molar mass from the mass of the unknown and the number of moles of unknown. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl. Then use the molality equation to calculate the moles of solute. What is a tefl qualification equivalent to.
 Source: pinterest.com
Source: pinterest.com
Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. First black basketball player in nba. Molal moles of solutekg of solvent moles of solute in 1 kg of water in the above example. Calculate the molar mass of the solute. Then use the molality equation to calculate the moles of solute.
 Source: dummies.com
Source: dummies.com
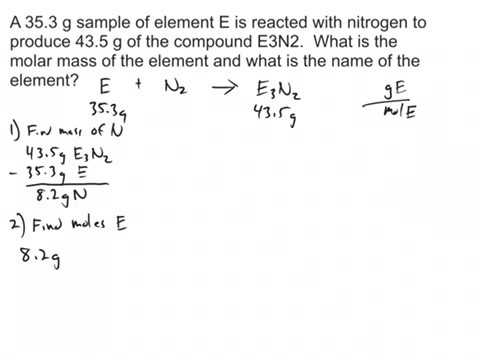
Write the atomic reaction showing the two reactants P4O10 and CaO on the left. How do I find the number of moles of an ion in a compound with an unknown cation. Then divide the grams of solute by the moles to determine the molar mass. Assuming the unknown is soluble in water dissolve 100 grams of the unknown in 1000 ml of water 1 kg of water. Determine the molar mass from the mass of the unknown and the number of moles of unknown.
 Source: youtube.com
Source: youtube.com
Using data given to us we first converted grams of released C O X 2 to moles. Determine the moles of unknown the solute from the molality of the solution and the mass of solvent in kilograms used to make the solution. Use the freeing point depression to calculate the molality of the solution. D T mK b or D T mK f. Then divide the grams of solute by the moles to determine the molar mass.
 Source: pinterest.com
Source: pinterest.com
Then here was the part I was confused on-. Calculate the molar mass of the solute. Since the sulfate has the formula M X 2 S O X 4 X 2 we know that we have the same amount of metal as sulfate. Assuming that the change in boiling point is 1 degree C the result becomes. How do I find the number of moles of an ion in a compound with an unknown cation.
 Source: pinterest.com
Source: pinterest.com
Lithium Sodium and Potassium as M. Calculate the molar mass of the solute. List the known quantities and plan the problem. Determine the percentage of each element present in the compound from the mass of each element present in a certain known mass of the compound. Determine the moles of unknown the solute from the molality of the solution and the mass of solvent in kilograms used to make the solution.
 Source: pinterest.com
Source: pinterest.com
Calculate the molar mass of the unknown compound. To calculate the molality of the solution. Calculate the molar mass of the solute. List the known quantities and plan the problem. How do you find the molar mass of an unknown compound.
 Source: pinterest.com
Source: pinterest.com
NaCl Na Cl. N S O X 4 m S O X 4 M S O X 4 635 m m o l. Then divide the grams of solute by the moles to determine the molar mass. List the known quantities and plan the problem. Determine the moles of unknown the solute from the molality of the solution and the mass of solvent in kilograms used to make the solution.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to find moles of unknown compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






