Your How to find moles of one atom in a compound images are available. How to find moles of one atom in a compound are a topic that is being searched for and liked by netizens today. You can Download the How to find moles of one atom in a compound files here. Find and Download all free vectors.
If you’re searching for how to find moles of one atom in a compound images information linked to the how to find moles of one atom in a compound keyword, you have pay a visit to the ideal site. Our website always gives you suggestions for viewing the maximum quality video and image content, please kindly surf and find more informative video content and images that match your interests.
How To Find Moles Of One Atom In A Compound. A mole of a substance or a mole of particles is defined as exactly 60221407610²³ particles which may be atoms molecules ions or electrons. There are three hydrogen atoms as indicated by the subscript. We now know that in every mole of adrenaline there are 48 mathrmg of oxygen. The molar mass of KNO3 is 391 140 3160 gmol 1011 gmol.
 Chemical Quantities Ppt Download From slideplayer.com
Chemical Quantities Ppt Download From slideplayer.com
If you have 1000 grams. A mole can be defined as the amount of substance. Meaning for every two atoms in a molecule of water there is one atom of hydrogen. Molecular mass 1 x 140067 3 x 100794 molecular mass 140067 302382. It can be expressed as grams liters atoms molecules or particles. N the number of moles of a compound.
From moles of a substance one can also find the number of atoms in a sample and vice versa.
And 1 mol of chlorine Cl has a mass of 35453 g the mass on the periodic table. Avogadros number is typically dimensionless but when it defines the mole it can be expressed as 602210 23 elementary entitiesmol. 48382 g x 1 mol 8006 g X mole SO3. Now the only confusing thing for me is the 5 moles. We also know that 1 mathrmmol of adrenaline weighs 183 mathrmg. In grams a mole is one formula mass.
 Source: slidetodoc.com
Source: slidetodoc.com
Next multiply the atomic mass of each atom by the number of atoms in the compound. One mole of a compound contains Avogadros number 6022 x 10 23 of molecules molecular compound or formula units ionic compoundThe molar mass of a compound tells you the mass of 1 mole of that substance. How many atoms of each element are found in. Molecular mass 1 x 140067 3 x 100794 molecular mass 140067 302382. How many moles of hydrogen atoms are there in 0046 g of C2H6O.
 Source: slideplayer.com
Source: slideplayer.com
Suppose that you have 500 g of KNO3. Since one mole of KOH contains one mole of K the answer is 602210 23 atoms of K. Then 1000 g 151001 gmol X g moles. Avogadros number is typically dimensionless but when it defines the mole it can be expressed as 602210 23 elementary entitiesmol. 48382 g x 1 mol 8006 g X mole SO3.
 Source: slideplayer.com
Source: slideplayer.com
Suppose that you have 500 g of KNO3. 5 moles H X 2 S O X 4. Next multiply the atomic mass of each atom by the number of atoms in the compound. There are 6022 10 23 atoms of potassium in every mole of potassium. Since one mole of KOH contains one mole of K the answer is 602210 23 atoms of K.
 Source: youtube.com
Source: youtube.com
In the same way HCl has one atom of Hydrogen H and one atom of Chlorine Cl. In grams a mole is one formula mass. It can be expressed as grams liters atoms molecules or particles. For example the compound water is made of hydrogen and oxygen with a 21 atomic ratio. Calculate Percentage Composition of Oxygen by Mass.
 Source: slideshare.net
Source: slideshare.net
And 1 mol of chlorine Cl has a mass of 35453 g the mass on the periodic table. We also know that 1 mathrmmol of adrenaline weighs 183 mathrmg. The subscripts in the formula indicate moles of each element per mole of compound. An atom is the smallest particle that can exist. There is one nitrogen atom no subscript is given for one atom.
 Source: wou.edu
Source: wou.edu
A mole of a substance or a mole of particles is defined as exactly 60221407610²³ particles which may be atoms molecules ions or electrons. Calculate Percentage Composition of Oxygen by Mass. So nNNa – n Na N number of atoms 2 1grmol H 2 moles H x 5 moles in the compound 10 moles. How many H atoms are there in 0046 g of C2H6OINTERVIEW1 Revell K. Suppose that you have 500 g of KNO3.
 Source: chemistry.wustl.edu
Source: chemistry.wustl.edu
NaCl 1 Sodium atom 1 Chlorine atom Determining the. Moles of element n moles elementone mole of compound K -. There are three hydrogen atoms as indicated by the subscript. Avogadros number is typically dimensionless but when it defines the mole it can be expressed as 602210 23 elementary entitiesmol. The bridge between atoms and moles is Avogadros number 602210 23.
 Source: youtube.com
Source: youtube.com
Everything is made from atoms. In 1 mole of SO3 there are 3 moles of oxygen atoms and moles of sulfur so x moles of SO3 will contain. Molecular mass 170305. And 1 mol of chlorine Cl has a mass of 35453 g the mass on the periodic table. There are 6022 10 23 atoms of potassium in every mole of potassium.
 Source: khanacademy.org
Source: khanacademy.org
If you had the compound sodium. For example one molecule of H2O has two atoms of Hydrogen H and one atom of Oxygen O. Method 1Method 1 of 2Calculating the Molar Mass of an Element. For example 1 mol of sodium Na has a mass of 229898 g the mass on the periodic table. How many atoms of each element are found in.
 Source: youtube.com
Source: youtube.com
If you had the compound sodium. KOH contains only one K so a mole of KOH contains one mole of K. Now the only confusing thing for me is the 5 moles. To calculate the mass of one mole of a compound take the molar mass which is the same as Atomic Mass of the elements used to make the compound and add them together. 5 moles H X 2 S O X 4.
 Source: dummies.com
Source: dummies.com
In grams a mole is one formula mass. In other words it tells you the number of. The mole is important because it allows chemists to work with the subatomic world with macro world units and amounts. Third find of positive charge atoms. There are 6022 10 23 atoms of potassium in every mole of potassium.
 Source: youtube.com
Source: youtube.com
From moles of a substance one can also find the number of atoms in a sample and vice versa. Everything is made from atoms. Then 1000 g 151001 gmol X g moles. For example the compound water is made of hydrogen and oxygen with a 21 atomic ratio. How is an atom different from a compound.
 Source: chem.fsu.edu
Source: chem.fsu.edu
And 1 mol of chlorine Cl has a mass of 35453 g the mass on the periodic table. I think you want the number of moles of positive ions so this will be moles of NH4 1811022 x 5 there are 5 atoms in NH4 9061022 Nmber of NH4 ions 181 x 10 22 ions and. So nNNa – n Na N number of atoms 2 1grmol H 2 moles H x 5 moles in the compound 10 moles. Everything is made from atoms. Then 1000 g 151001 gmol X g moles.
 Source: slideplayer.com
Source: slideplayer.com
How many atoms of each element are found in. There is one nitrogen atom no subscript is given for one atom. X mole SO3 x 3 mol Oxygen atom 1 mole SO3 Z mole of oxygen atoms. For example 1 mol of sodium Na has a mass of 229898 g the mass on the periodic table. 10 moles H X 2 602 10 23 602 10 23 atoms.
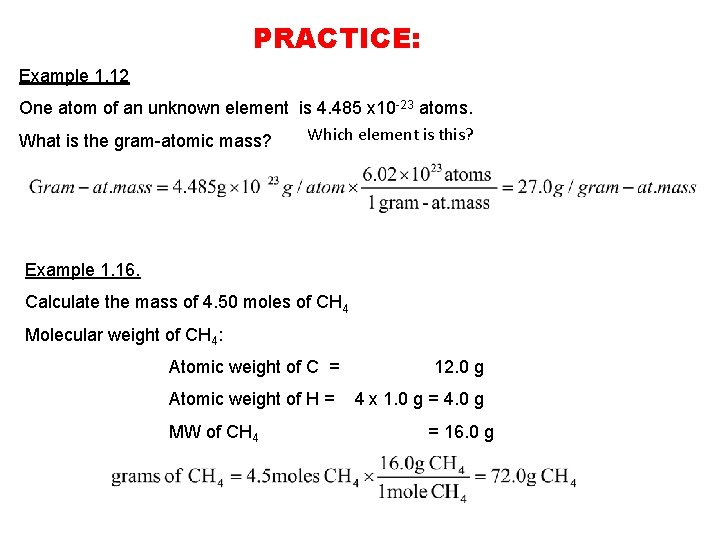 Source: slidetodoc.com
Source: slidetodoc.com
Therefore there are 500 g 1011 gmol 00495 mol of KNO3. How many moles of hydrogen atoms are there in 0046 g of C2H6O. For example one molecule of H2O has two atoms of Hydrogen H and one atom of Oxygen O. NaCl 1 Sodium atom 1 Chlorine atom Determining the. Answer 1 of 3.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find moles of one atom in a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






