Your How to find moles of ions in a compound images are ready. How to find moles of ions in a compound are a topic that is being searched for and liked by netizens today. You can Find and Download the How to find moles of ions in a compound files here. Download all free photos and vectors.
If you’re looking for how to find moles of ions in a compound images information linked to the how to find moles of ions in a compound interest, you have visit the right blog. Our site always provides you with suggestions for seeing the highest quality video and image content, please kindly hunt and locate more enlightening video content and graphics that fit your interests.
How To Find Moles Of Ions In A Compound. To acquire the variety of moles divide the mass of compound by the molar mass of the compound expressed in grams. The mole abbreviated mol is an SI unit which measures the number of particles in a specific. So the correct answer is a. The cation right here is Sr2 and also the anion is F-.
 Formula Units Number Of Ions Calculations Youtube From youtube.com
Formula Units Number Of Ions Calculations Youtube From youtube.com
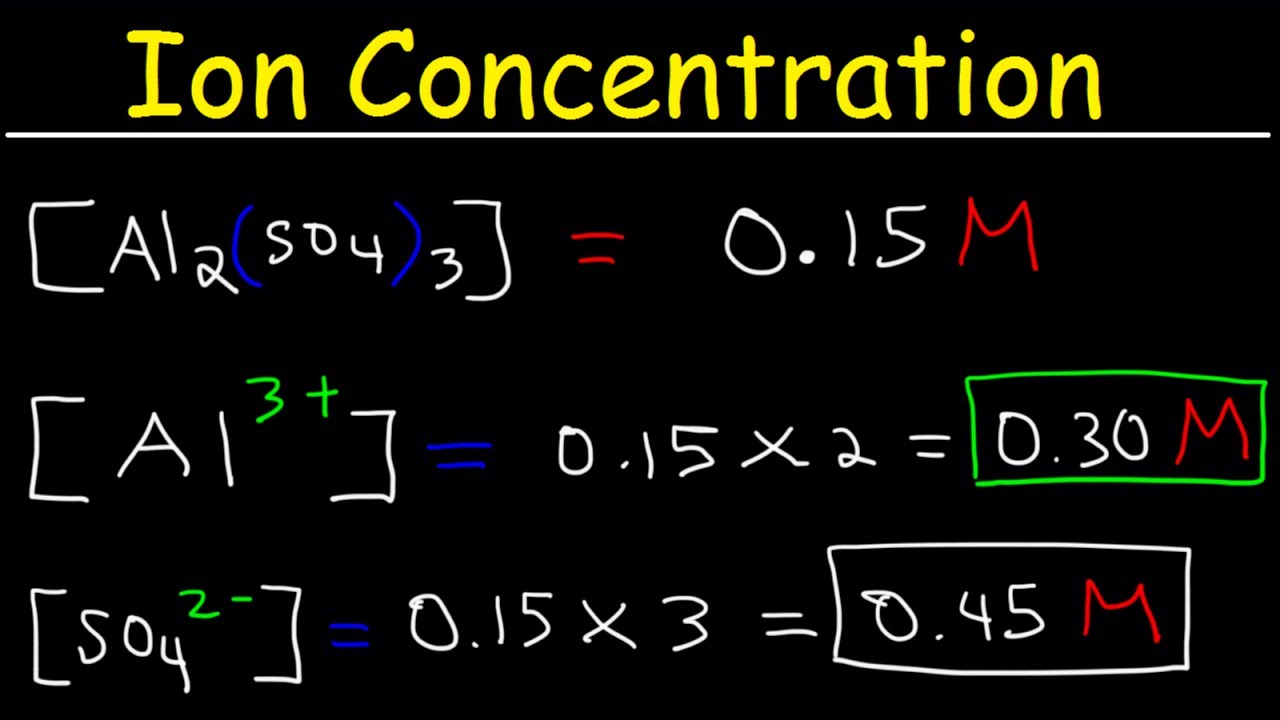
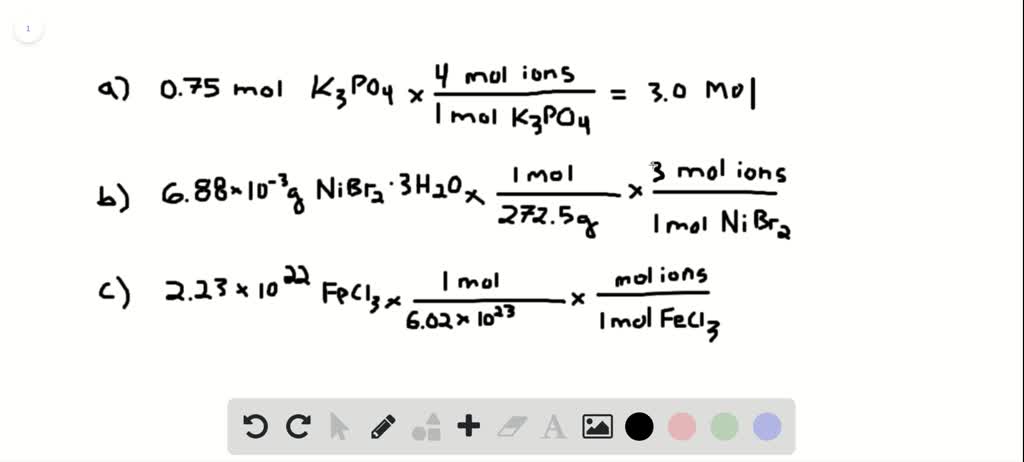
A mole of a substance or a mole of particles is defined as exactly 60221407610²³ particles which may be atoms molecules ions or electrons. For every mole of MgI2 there is one mole of Mg and 2 moles of I- moles of MgI2 moles of Mg. From the periodic table. There room 2 F- ions for every one Sr2 ion so that the all at once charge is neutral. To find the molarity of the ions first determine the molarity of the solute and the ion-to-solute ratio. This procedure is used to determine the number of moles of nickel ions in your compound.
Specifically 1 mole represents 6022 x 1023 atoms or molecules of substance.
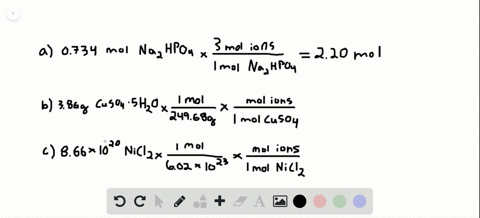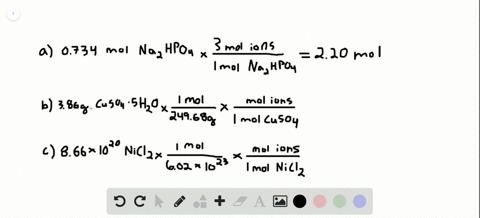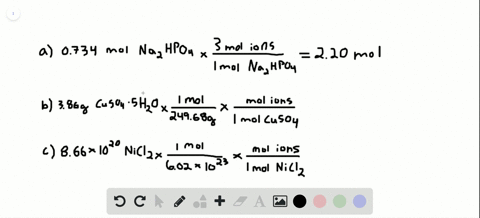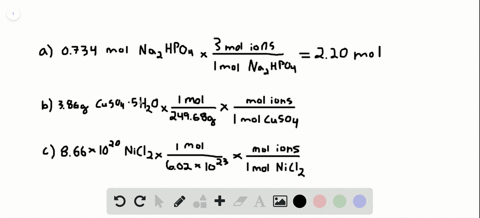
Number of ions left textnumber of moles righttextX left N_A right where N_A Avogadros number. Nov 09 2017 So in every components unit there are 3 sodium ions and 1 phosphate ion. To acquire the variety of moles divide the mass of compound by the molar mass of the compound expressed in grams. Do not use the GoLink with the spectrometer. That cancels out the grams of Mg OH2 and leaves you with moles of Mg OH2. So in 1 mole of sodium phosphate there will be 3 mole of sodium ions and 1 mole of phosphate ions.
 Source: youtube.com
Source: youtube.com
So in 1 mole of sodium phosphate there will be 3 mole of sodium ions and 1 mole of phosphate ions. To go from moles of an ionic compound to the number of ions youmust multiply your number of moles by Avogadros number Avogarosnumber is approximately 602 1023. 10 moles of sodium ions 10 6022 10 23 6022 10 24 sodium ions. 1 molar mass of Mg OH2. The spectrometer needs to be powered for about 5 minutes before using so do this step before preparing your solutions.
 Source: youtube.com
Source: youtube.com
There room 2 F- ions for every one Sr2 ion so that the all at once charge is neutral. Number moles O 13916 00868. Finally combine the two ions to form an electrically neutral compound. The mole is important because it allows chemists to work with the subatomic world with macro world units and amounts. NaCl Na Cl.
 Source: youtube.com
Source: youtube.com
NaClaq Na aq Cl aq So if every mole of sodium chloride produces One mole of sodium cations it follows that the number of moles of sodium cation present in your solution will be equal to the number of moles of sodium chloride you dissolved to create this solution. To find the molarity of the ions first determine the molarity of the solute and the ion-to-solute ratio. Na3PO4 which is Na3PO43-. So the first thing you want to do is calculate the molar mass of magnesium hydroxide then you multiply the 5 grams of magnesium hydroxide by. Now to calculate the variety of ions present.
 Source: numerade.com
Source: numerade.com
The mathematical equation N n N A can be used to find the number of atoms ions or molecules in any amount in moles of atoms ions or molecules. Then using the mass given and molar massive to discover the number of moles the SrF2 present. So the first thing you want to do is calculate the molar mass of magnesium hydroxide then you multiply the 5 grams of magnesium hydroxide by. Write the atomic reaction showing the two reactants P4O10 and CaO on the left. So the correct answer is a.
 Source: numerade.com
Source: numerade.com
Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. For every mole of MgI2 there is one mole of Mg and 2 moles of I- moles of MgI2 moles of Mg. NaCl Na Cl. So in each formula unit there are 3 sodium ions and 1 phosphate ion. Then identify the anion and write down its symbol and charge.
 Source: clutchprep.com
Source: clutchprep.com
NaClaq Na aq Cl aq So if every mole of sodium chloride produces One mole of sodium cations it follows that the number of moles of sodium cation present in your solution will be equal to the number of moles of sodium chloride you dissolved to create this solution. The spectrometer needs to be powered for about 5 minutes before using so do this step before preparing your solutions. Now to calculate the variety of ions present. 1 molar mass of Mg OH2. The mole represents a quantity of substance but relates to the number of atoms or molecules rather than mass or volume.
 Source: khanacademy.org
Source: khanacademy.org
The cation right here is Sr2 and also the anion is F-. Finally combine the two ions to form an electrically neutral compound. From the periodic table. 10 moles of sodium ions 10 6022 10 23 6022 10 24 sodium ions. Na3PO4 which is Na3PO43-.
 Source: youtube.com
Source: youtube.com
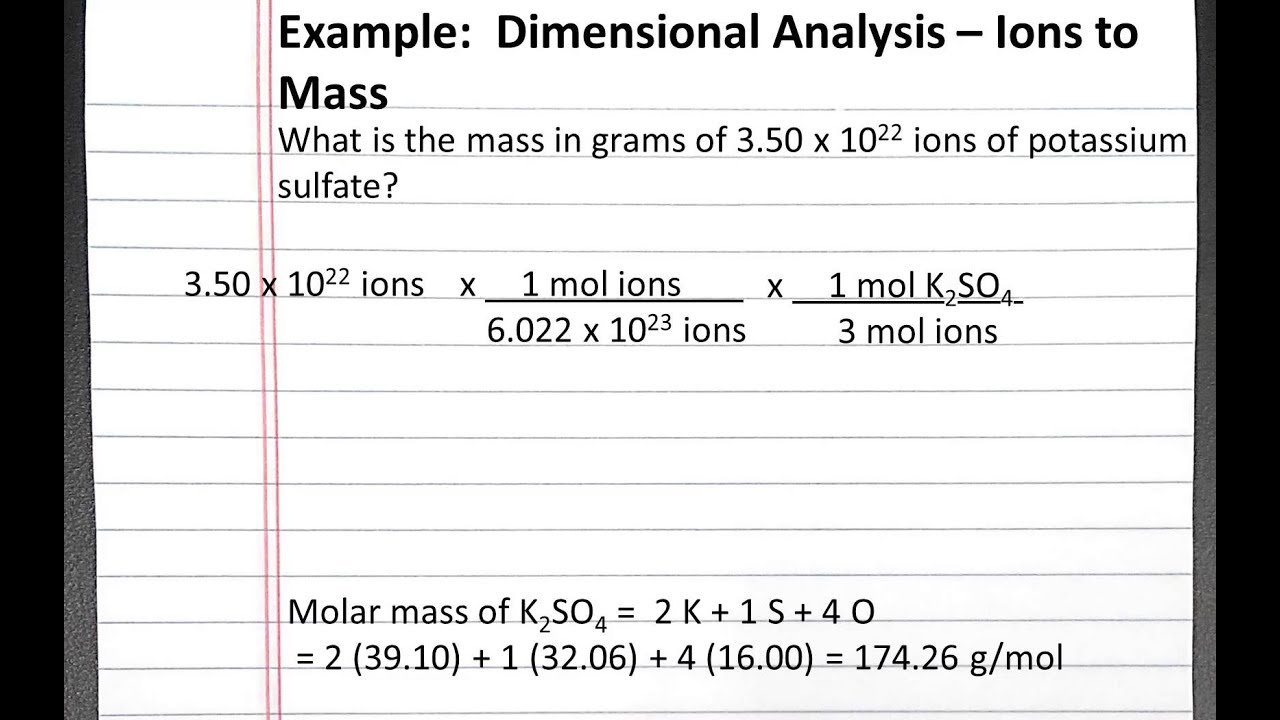
In 100 formula units there are 300 sodium ions and 100 phosphate ions. The mole is important because it allows chemists to work with the subatomic world with macro world units and amounts. Atomic mass of Cu 6355. So in 1 mole of sodium phosphate there will be 3 mole of sodium ions and 1 mole of phosphate ions. In this video well walk through this process for the ionic compound calcium bromide.
 Source: youtube.com
Source: youtube.com
10 moles of sodium ions 10 6022 10 23 6022 10 24 sodium ions. NaClaq Na aq Cl aq So if every mole of sodium chloride produces One mole of sodium cations it follows that the number of moles of sodium cation present in your solution will be equal to the number of moles of sodium chloride you dissolved to create this solution. Finally combine the two ions to form an electrically neutral compound. The mole abbreviated mol is an SI unit which measures the number of particles in a specific. Formula to calculate moles.
 Source: youtube.com
Source: youtube.com
Formula to calculate moles. 10 moles of helium atoms 10 6022 10 23 6022 10 24 helium atoms. Now to calculate the variety of ions present. The mole abbreviated mol is an SI unit which measures the number of particles in a specific. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl.
 Source: slideplayer.com
Source: slideplayer.com
To find the molarity of the ions first determine the molarity of the solute and the ion-to-solute ratio. More specifically you know that you have. To find the formula of an ionic compound first identify the cation and write down its symbol and charge. In this video well walk through this process for the ionic compound calcium bromide. From compound to compound to a number of atoms in a molecule vary.
 Source: youtube.com
Source: youtube.com
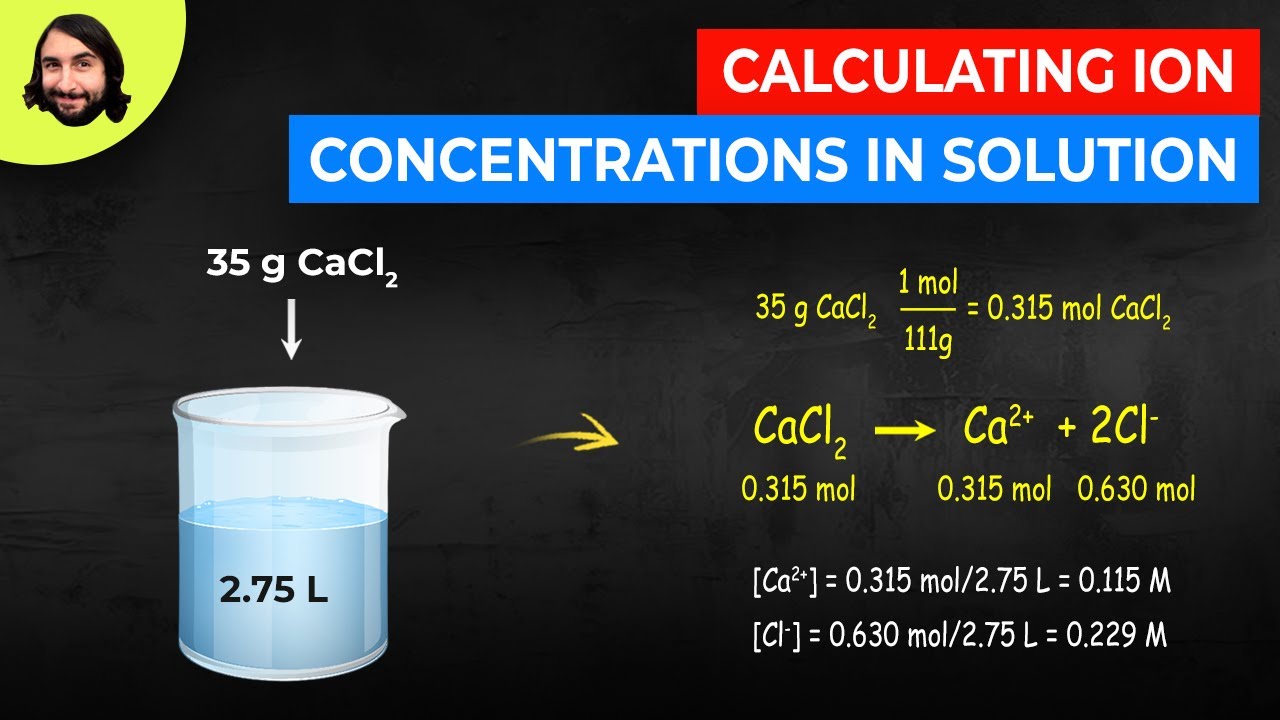
Finally combine the two ions to form an electrically neutral compound. Write the atomic reaction showing the two reactants P4O10 and CaO on the left. This worked example problem illustrates the steps necessary to calculate the concentration of ions in an aqueous solution in terms of molarityMolarity is one of the most common units of concentration. Number of ions left textnumber of moles righttextX left N_A right where N_A Avogadros number. In this video well walk through this process for the ionic compound calcium bromide.
 Source: numerade.com
Source: numerade.com
Atomic mass of Cu 6355. So in each formula unit there are 3 sodium ions and 1 phosphate ion. Atomic mass of CuCl 2 1 6355 2 3545 Atomic mass of CuCl 2 6355 709. You calculate the number of moles by dividing the mass of substance by the substances atomic or molecular weight. For every mole of MgI2 there is one mole of Mg and 2 moles of I- moles of MgI2 moles of Mg.
 Source: youtube.com
Source: youtube.com
NaCl 229898 gL 354530 gL. Take this number of moles and multiply it by avogadros number. Formula to calculate moles. How do I find the number of moles of an ion in a compound with an unknown cation. The mole represents a quantity of substance but relates to the number of atoms or molecules rather than mass or volume.
 Source: slideplayer.com
Source: slideplayer.com
Atomic mass of Cu 6355. NaClaq Na aq Cl aq So if every mole of sodium chloride produces One mole of sodium cations it follows that the number of moles of sodium cation present in your solution will be equal to the number of moles of sodium chloride you dissolved to create this solution. For example one molecule of H2O has two atoms of Hydrogen H. Atomic mass of CuCl 2 1 6355 2 3545 Atomic mass of CuCl 2 6355 709. The spectrometer needs to be powered for about 5 minutes before using so do this step before preparing your solutions.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find moles of ions in a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.