Your How to find moles of hydrogen per g sample images are available. How to find moles of hydrogen per g sample are a topic that is being searched for and liked by netizens today. You can Find and Download the How to find moles of hydrogen per g sample files here. Download all free images.
If you’re looking for how to find moles of hydrogen per g sample pictures information connected with to the how to find moles of hydrogen per g sample keyword, you have visit the right site. Our website always provides you with hints for seeing the highest quality video and picture content, please kindly search and find more enlightening video articles and images that match your interests.
How To Find Moles Of Hydrogen Per G Sample. 100 grams Hydrogen to mol. Molar mass of Hydrogen Peroxide mol. Al 100 100 Al. Since the Al and the Zn must add up to be 100 ie Zn 100 Al equation 11 can be rewritten as.
 Calculate The Concentration Of Nitric Acid In Moles Per Litre In A Sample Which Has A Density 1 41 G Ml 1 And The Mass Per Cent Of Nitric Acid In It Being 69 From toppr.com
Calculate The Concentration Of Nitric Acid In Moles Per Litre In A Sample Which Has A Density 1 41 G Ml 1 And The Mass Per Cent Of Nitric Acid In It Being 69 From toppr.com
6022 10 23 is known as the Avogadro Number or Avogadro Constant and is given the symbol N A 1. 1 Given mass of H2 gas 29 g Number of moles of H2 present in this sample Given massMolar mass 292 145 mol Since number of moles of. 1008 amu x 1661 x10-24 gamu 1674 x10-24 g Mass of 1 mole of H atoms. 50 grams Hydrogen to mol 4960613 mol. Al 100 100 Al. 1 mole of any substance contains 6022 10 23 particles.
If you were to use H in one gram of Hydrogen gas there will be.
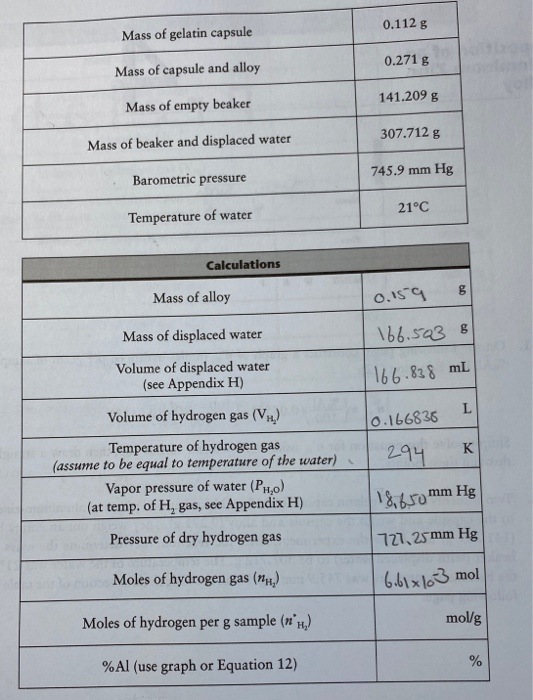
The number of moles of hydrogen produced per gram of sample can be calculated from. Al 100 100 Al. So we could write it this way. So the molar mass of glucose is going to be six times the molar mass of carbon plus 12 times the molar mass of hydrogen plus six times the molar mass of oxygen. 100 grams Hydrogen to mol. Molarity 10-6 mol L-1.
 Source: nagwa.com
Source: nagwa.com
So we could write it this way. N 100 Al 00556 100 Zn 00153 Hydrogen b l gb l g11 where n Hydrogen is the number of moles of hy-drogen per gram of sample. So the molar mass of glucose is going to be six times the molar mass of carbon plus 12 times the molar mass of hydrogen plus six times the molar mass of oxygen. 6022 10 23 is known as the Avogadro Number or Avogadro Constant and is given the symbol N A 1. The molecular formula of methane CH 4 suggests that one molecule of this compound consists of 4 atoms of H.
 Source: youtube.com
Source: youtube.com
In the same way HCl has one atom of Hydrogen H and one atom of Chlorine Cl. 6022 10 23 is known as the Avogadro Number or Avogadro Constant and is given the symbol N A 1. Well we know that each mole has roughly 6022 times 10 to the 23rd molecules in it so we just have to multiply this times 6022 times 10 to the 23rd. Molecular weight of hydrogen gas2016gm. 306x10³ g 75 gmol 408x10⁵ mol C₂H₅NO₂.
 Source: study.com
Source: study.com
Use the mass of the hydrogen gas to calculate the gas moles directly. Alternatively you can first find the number of moles of water in get in that 235 g sample. 20 grams Hydrogen to mol 1984245 mol. For example one molecule of H2O has two atoms of Hydrogen H and one atom of Oxygen O. 1 grams Hydrogen to mol 099212 mol.
 Source: youtube.com
Source: youtube.com
In using H2 in one gram of Hydrogen gas there will be 0496 moles. The number of moles of hydrogen produced per gram of sample can be calculated from. N n 6022 10 23. In using H2 in one gram of Hydrogen gas there will be 0496 moles. 5 grams Hydrogen to mol 496061 mol.
 Source: chegg.com
Source: chegg.com
If you were to use H in one gram of Hydrogen gas there will be. 1 grams Hydrogen Peroxide 0029399071224542 mole using the molecular weight calculator and the molar mass of H2O2. Therefore NaCl 584538 gL. Find the moles of H. So for the above compound were looking at 177035 grams per mol.
 Source: khanacademy.org
Source: khanacademy.org
Moles molarity mol L-1 volume L Calculate moles of H. Mass of H 2000 g 0075368843 150737686 g. N 100 Al 00556 100 Zn 00153 Hydrogen b l gb l g11 where n Hydrogen is the number of moles of hy-drogen per gram of sample. 1 grams Hydrogen Peroxide 0029399071224542 mole using the molecular weight calculator and the molar mass of H2O2. To calculate the number of.
 Source: toppr.com
Source: toppr.com
10 grams Hydrogen to mol 992123 mol. From compound to compound to a number of atoms in a molecule vary. Since your sample has a mass of 235 g it follows that it will contain. 306x10³ g 75 gmol 408x10⁵ mol C₂H₅NO₂. NaCl 229898 gL 354530 gL.
 Source: khanacademy.org
Source: khanacademy.org
From compound to compound to a number of atoms in a molecule vary. Use the mass of the hydrogen gas to calculate the gas moles directly. Well we know that each mole has roughly 6022 times 10 to the 23rd molecules in it so we just have to multiply this times 6022 times 10 to the 23rd. Mass of H 2000 g 0075368843 150737686 g. 5 grams Hydrogen to mol 496061 mol.
 Source: youtube.com
Source: youtube.com
Since the Al and the Zn must add up to be 100 ie Zn 100 Al equation 11 can be rewritten as. So this means that 45 grams of this makes 254187 mols. N 100 Al 00556 100 Zn 00153 Hydrogen b l gb l g11 where n Hydrogen is the number of moles of hy-drogen per gram of sample. N n 6022 10 23. Well we know that each mole has roughly 6022 times 10 to the 23rd molecules in it so we just have to multiply this times 6022 times 10 to the 23rd.
 Source: quizlet.com
Source: quizlet.com
So the number of moles of H atom in it. Well we know that each mole has roughly 6022 times 10 to the 23rd molecules in it so we just have to multiply this times 6022 times 10 to the 23rd. If you were to use H in one gram of Hydrogen gas there will be. Since the Al and the Zn must add up to be 100 ie Zn 100 Al equation 11 can be rewritten as. 4 52 1024 Avogadros number.
 Source: youtube.com
Source: youtube.com
Al 100 100 Al. 2016 gm H2 gas1 mole of H2 gas. So for example H2O is 100710071599918013 grams. Molar mass of NH43AsO3 is calculated by adding all the atomic weights of the composite atoms and changing the unit to grams. 150737686 g 1008 gmol 149541355 mol.

235g water 1119 g H 100g water 263 g H. Now the next question is how many molecules is that. 235g water 1119 g H 100g water 263 g H. So for example H2O is 100710071599918013 grams. The molecular formula of methane CH 4 suggests that one molecule of this compound consists of 4 atoms of H.
 Source: scholr.com
Source: scholr.com
In order to calculate the number of hydrogen moles we multiply the number of glycine moles by. 5 grams Hydrogen to mol 496061 mol. 1 grams Hydrogen to mol 099212 mol. One mole of O atoms corresponds to 159994 g Two moles of H atoms corresponds to 2 x 10079 g Sum molar mass 180152 g H 2O per mole Chapter 3 Calculation of Molar Masses Calculate the molar mass of the following Magnesium nitrate MgNO 32 1 Mg 243050 2 N 2x 140067 280134 6 O 6 x 159994 959964 Molar mass of MgNO 32 148. Molarity moles volume L Rearrange this equation formula to find moles.
 Source: wikihow.com
Source: wikihow.com
50 grams Hydrogen to mol 4960613 mol. In the same way HCl has one atom of Hydrogen H and one atom of Chlorine Cl. 235g 1 mole H2O 180153g 13044 moles H2O. Mass of H 2000 g 0075368843 150737686 g. 4 52 1024 Avogadros number.
 Source: in.pinterest.com
Source: in.pinterest.com
So for example H2O is 100710071599918013 grams. In saying Hydrogen gas I am assuming you are using H2 and not just H as that is how it should be done. In using H2 in one gram of Hydrogen gas there will be 0496 moles. For example 250 grams g of the hydrogen gas corresponds to 250 g 2 gmole 125 moles. Now the next question is how many molecules is that.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find moles of hydrogen per g sample by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






