Your How to find moles of hydrogen images are available. How to find moles of hydrogen are a topic that is being searched for and liked by netizens today. You can Find and Download the How to find moles of hydrogen files here. Download all free photos and vectors.
If you’re looking for how to find moles of hydrogen images information related to the how to find moles of hydrogen interest, you have pay a visit to the right blog. Our website frequently gives you suggestions for seeking the maximum quality video and image content, please kindly search and locate more informative video articles and graphics that fit your interests.
How To Find Moles Of Hydrogen. Make sure you complete table headings and units Hint. Calculate moles of H. Mg 2 HCI MgC12 Ha It is apparent that each mole of Mg reacted produces one mole of hydrogen gas. Volume 100 mL 100 10-3 010 L moles H 10-6 010 10-7 mol Calculate moles of H.
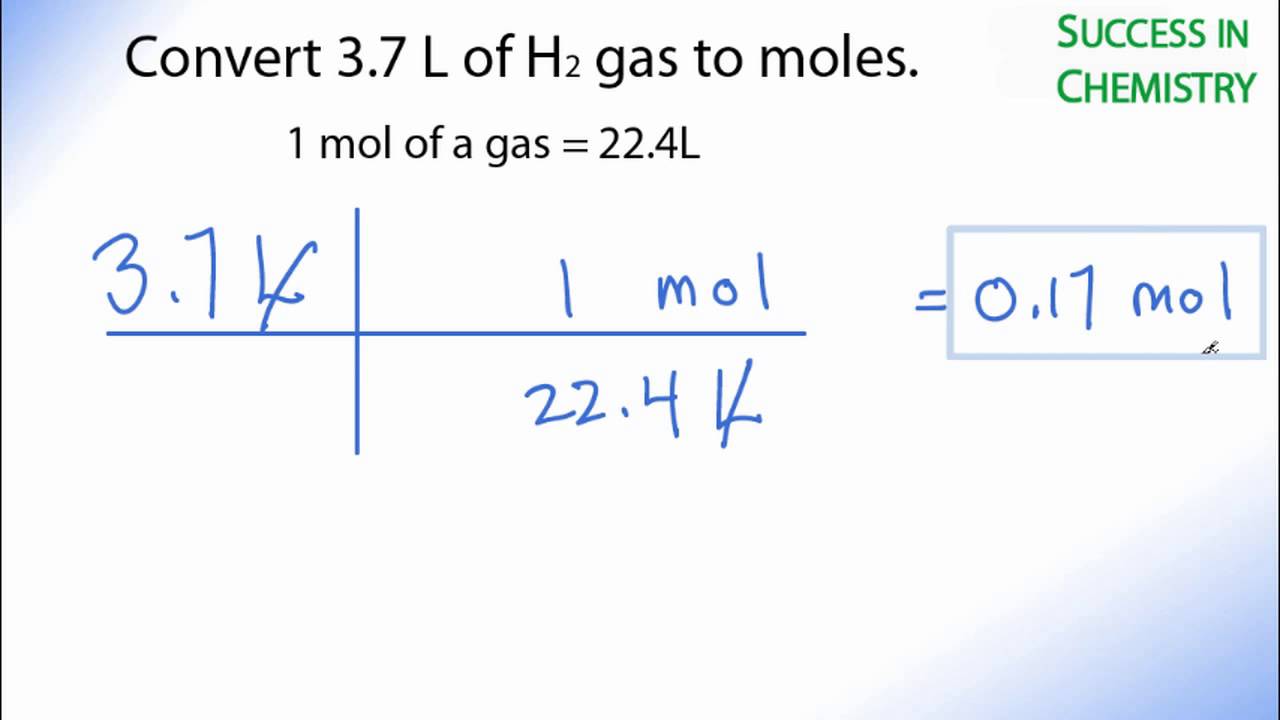 Convert 3 7 L Of H2 Gas To Moles Youtube From youtube.com
Convert 3 7 L Of H2 Gas To Moles Youtube From youtube.com
Mg 2 HCI MgC12 Ha It is apparent that each mole of Mg reacted produces one mole of hydrogen gas. 20 grams Hydrogen to mol 1984245 mol. Weight to volume volume to weight price mole to volume and weight mass and molar concentration density. 2 moles NH 3 3 moles H 2 2 moles NH 3 6 eggs 3 moles H 2 800 moles H 2 533 moles NH 3 3 1 bag flour. HINTUse the balanced equation provided in the lab introduction. Then use this value to calculate the number of moles of hydrogen peroxide you began the experiment with.
50 grams Hydrogen to mol 4960613 mol.
In the example the amount of hydrogen is 202650 x 0025 29315 x 8314472 2078 moles. 1 000 000 000 000. Density of hydrogen is equal to 0082 kgm³. Mg 2 HCI MgC12 Ha It is apparent that each mole of Mg reacted produces one mole of hydrogen gas. Determine the pressure of the dry gas by correcting for water vapor and differences in water levels. 40 grams Hydrogen to mol 396849 mol.
 Source: youtube.com
Source: youtube.com
1 grams Hydrogen to mol 099212 mol. 5 grams Hydrogen to mol 496061 mol. CAS Registry Number CAS RN. The molecular formula for Hydrogen Peroxide is H2O2. Here are the steps.
 Source: pinterest.com
Source: pinterest.com
In the example the amount of hydrogen is 202650 x 0025 29315 x 8314472 2078 moles. Hydrogen H Oxygen O Molecular weight. Quick conversion chart of grams Hydrogen to mol. Weight to volume volume to weight price mole to volume and weight mass and molar concentration density. 1 mole is equal to 1 moles Hydrogen Peroxide or 3401468 grams.
 Source: pinterest.com
Source: pinterest.com
Charge of electron 160 x 10 -19 coulombs. Calculate the number of hydrogen ions. 1 mole Avogadros Number of particles. 30 grams Hydrogen to mol 2976368 mol. 1 mole of electrons contains 602 x 10 23 Avogadros No.
 Source: youtube.com
Source: youtube.com
Calculate the number of moles of O2 produced using the ideal gas law. So there will be a total of 60210232121024 hydrogen atoms. Determine the volume of the dry gas at STP. Molecular weight of Hydrogen Peroxide or grams. HINTUse the balanced equation provided in the lab introduction.
 Source: pinterest.com
Source: pinterest.com
How many moles of ammonia are produced from 800 mol of hydrogen reacting with nitrogen. In the example the amount of hydrogen is 202650 x 0025 29315 x 8314472 2078 moles. After that we can now calculate the number of moles of hydrogen in the compound using the stoichiometric ratio 22 moles H 1 mole C12H22O11 22 m o l e s H 1 m o l e C 12 H 22 O 11. The 18 grams of hydrogen gas is equal to 18 2 9 moles of hydrogen two. Therefore 3 moles of hydrogen gas contains 2 x 3 x 6022 x 1023 hydrogen atoms or 36132 x 1024.
 Source: pinterest.com
Source: pinterest.com
Volume 100 mL 100 10-3 010 L moles H 10-6 010 10-7 mol Calculate moles of H. 50 grams Hydrogen to mol 4960613 mol. In respect to this how many hydrogen atoms are there in 300 mole of h2o. Also to do this you need to know the volume of the solution and how many solutes has been dissolved in the solution. You can calculate how many H2 atoms are in 5 moles of H2O by using Avogadros number.
 Source: pinterest.com
Source: pinterest.com
PvnRT 100 025L n 00821 L x atmmol x K 292 K 025L 2397 001 M O2. 1 grams Hydrogen to mol 099212 mol. The SI base unit for amount of substance is the mole. Since water has a chemical formula of H2O there will be 2 moles of hydrogen in every mole of water. Therefore the molarity is 300 g3401 gmol 882 M.
 Source: pinterest.com
Source: pinterest.com
The 18 grams of hydrogen gas is equal to 18 2 9 moles of hydrogen two. In one mole of water there will exist approximately 6021023 water molecules. 1 mole is equal to 1 moles Hydrogen Peroxide or 3401468 grams. The equation for this half-reaction is. Calculate the number of hydrogen ions.
 Source: wikihow.com
Source: wikihow.com
Since water has a chemical formula of H2O there will be 2 moles of hydrogen in every mole of water. Remember at STP 1 mole of any gas occupies 224 L Write the equation for the half-reaction that takes place. In one mole of water there will exist approximately 6021023 water molecules. Record the volume on the left side of the plunger Mass Volume variable mL g variable 1 43 1 0047 1 2 0021 19 86 3 0089 4 006 57 66 5 007 163 6 0178 1. 5 moles2because hydrogen is diatomic60231023 atoms 60221024 Hydrogen atoms.
 Source: youtube.com
Source: youtube.com
40 grams Hydrogen to mol 396849 mol. Determine the volume of the dry gas at STP. Calculate the number of moles of H 2. The equation for this half-reaction is. 1 mole is equal to 1 moles Hydrogen Peroxide or 3401468 grams.

Therefore 3 moles of hydrogen gas contains 2 x 3 x 6022 x 1023 hydrogen atoms or 36132 x 1024. Then use this value to calculate the number of moles of hydrogen peroxide you began the experiment with. Here are the steps. Remember at STP 1 mole of any gas occupies 224 L Write the equation for the half-reaction that takes place. 2 moles NH 3 3 moles H 2 2 moles NH 3 6 eggs 3 moles H 2 800 moles H 2 533 moles NH 3 3 1 bag flour.
 Source: brainly.in
Source: brainly.in
In the example the amount of hydrogen is 202650 x 0025 29315 x 8314472 2078 moles. How to find moles in the solution is to calculate how many molecules the solution contains. In respect to this how many hydrogen atoms are there in 300 mole of h2o. Multiply the volume and pressure and divide the product by the temperature and the molar gas constant to calculate moles of the hydrogen gas. Calculate the number of moles of hydrogen gas produced.
 Source: youtube.com
Source: youtube.com
Make sure you complete table headings and units Hint. 5 grams Hydrogen to mol 496061 mol. The 18 grams of hydrogen gas is equal to 18 2 9 moles of hydrogen two. Mg 2 HCI MgC12 Ha It is apparent that each mole of Mg reacted produces one mole of hydrogen gas. 1 mole of electrons contains 602 x 10 23 Avogadros No.
 Source: pinterest.com
Source: pinterest.com
Calculate the moles of hydrogen gas left column generated for each trial. At 0C 32F or 27315K at standard atmospheric pressure. Hydrogen weighs 0000082 gram per cubic centimeter or 0082 kilogram per cubic meter ie. Calculate the number of moles of H 2. 10 grams Hydrogen to mol 992123 mol.
 Source: youtube.com
Source: youtube.com
Weight to volume volume to weight price mole to volume and weight mass and molar concentration density. Remember at STP 1 mole of any gas occupies 224 L Write the equation for the half-reaction that takes place. That means the 1 mole of electrons carry 602 x 10 23 x 160 x 10. Calculate moles of H. The molarity is obtained as moles of solute in 1 L 1000 mL of solution.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to find moles of hydrogen by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






