Your How to find moles of anhydrous compound images are ready in this website. How to find moles of anhydrous compound are a topic that is being searched for and liked by netizens now. You can Find and Download the How to find moles of anhydrous compound files here. Get all free vectors.
If you’re looking for how to find moles of anhydrous compound images information connected with to the how to find moles of anhydrous compound keyword, you have pay a visit to the ideal site. Our website always provides you with hints for downloading the highest quality video and image content, please kindly surf and find more enlightening video articles and images that fit your interests.
How To Find Moles Of Anhydrous Compound. The answers will not be exact but enough to tell the formula. Lastly to find the number of moles of water per mole of hydrate I divided the number of moles of anhydrous salt remaining by itself and by the number of moles of water lost. Determine the grams of anhydrate remaining in the crucible. How do you find the moles of anhydrous CuSO4.
 Calculating Molar Mass And Number Of Moles Worked Example Video Khan Academy From khanacademy.org
Calculating Molar Mass And Number Of Moles Worked Example Video Khan Academy From khanacademy.org
Cu 1 6355 6355. Divide the mass of water lost when you heated the salt by the molar mass of water roughly 18 grams per mole. Divide the mass of your anhydrous heated salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. This is how many moles of anhydrous sodium carbonate dissolved. It contains plenty of examples and practice problemsMy E-Book. Divide the moles of water by the moles of salt to find the ratio.
Convert this to moles of water by dividing by the molar mass of water which is 1802 gmol.
Determine the grams of anhydrate remaining in the crucible. The symbol represents heat. Subtract the Constant Weight of the Empty Crucible from the Constant Weight After Heating 19. Divide the mass of your anhydrous heated salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. Divided the grams of anhydrate by its molar mass to find the number of moles 20. Enter a chemical formula to calculate its molar mass and elemental composition.

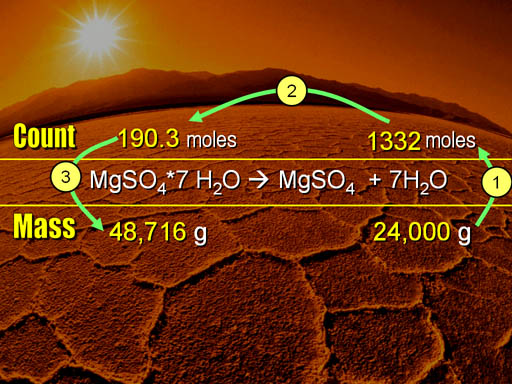
482g 1 mole MgSO4 12037g 004004 moles MgSO4. Determine the grams of anhydrate remaining in the crucible. To do this we divide the mass of anhydrate by the molar mass of anhydrate to get the moles of anhydrate. Convert this to moles of water by dividing by the molar mass of water which is 1802 gmol. Answered 2 years ago Author has 17K answers and 7M answer views.
 Source: chem.libretexts.org
Source: chem.libretexts.org
In our example 16 grams 160 grams per mole 01 moles. Divide moles of water by the moles of anhydrate to get the mole ratio. Divide the mass of water lost when you heated the salt by the molar mass of water roughly 18 grams per mole. Click to see full answer. Enter a chemical formula to calculate its molar mass and elemental composition.
 Source: chegg.com
Source: chegg.com
Divide the mass of your anhydrous heated salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. Well take the mass of the hydrate subject it to PROLONGED drying and then measure the mass lost while drying. 151 g Na2 CO3 106 gmol Na2 CO3 0142 moles Na2 CO3. Enter a chemical formula to calculate its molar mass and elemental composition. The answers will not be exact but enough to tell the formula.
 Source: socratic.org
Source: socratic.org
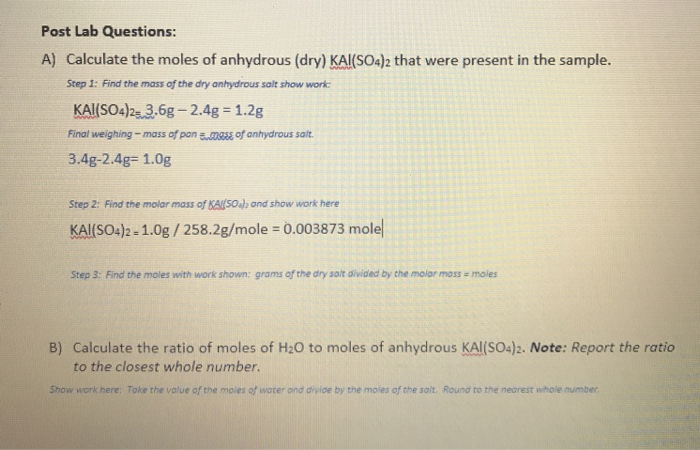
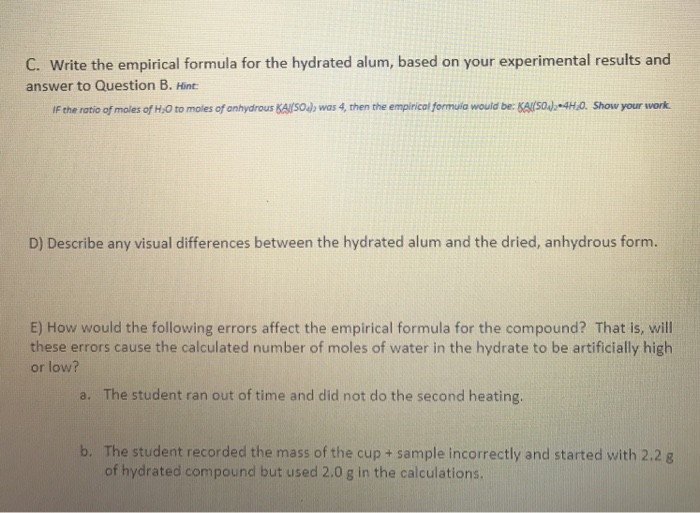
What I did was find the formula mass of which turned out to be 18757g. For example gaseous ammonia is called anhydrous ammonia to distinguish it from its aqueous formGaseous hydrogen chloride is called anhydrous hydrogen chloride to distinguish it from hydrochloric acid. Find the molar mass of KAKSO2 and show work here KAlSO42 - 10g 2582gmole 0003873 mole Step 3. I performed that same operation to find the number of moles of water lost using a molar mass of 1801528 gmol for H 2 O. In other words the if 4875g of the hydrated compound is used.

7 Determine smallest whole-number ratio between sodium carbonate and water. Capitalize the first letter in chemical symbol and use lower case for the remaining letters. Lastly to find the number of moles of water per mole of hydrate I divided the number of moles of anhydrous salt remaining by itself and by the number of moles of water lost. Hydrate —— anhydrous salt water. Now the difference between the mass of the hydrate and the mass of the anhydrous salt will be equal to the mass of water of hydration.
 Source: studylib.net
Source: studylib.net
Molar mass of MgSO4anhydrous is 1203676 gmol Convert between MgSO4anhydrous weight and moles. Convert the mass of the anhydrous salt m2 by dividing it by the molar mass of the anhydrous salt. O 6 1600 9600. Divide the mass of anhydrate by the molar mass of anhydrate to get moles of anhydrate. The number of moles of water combined with one mole of anhydrous magnesium sulfate can be determined and ultimately the formula for the hydrate.
 Source: chegg.com
Source: chegg.com
Divide moles of water by the moles of anhydrate to get the mole ratio. This is how many moles of anhydrous sodium carbonate dissolved. Divide moles of water by the moles of anhydrate to get the mole ratio. Lastly to find the number of moles of water per mole of hydrate I divided the number of moles of anhydrous salt remaining by itself and by the number of moles of water lost. You can thus say that the hydrate contained.
 Source: chegg.com
Source: chegg.com
Grams of the dry salt divided by the molar mass moles B Calculate. Grams of the dry salt divided by the molar mass moles B Calculate. 1 Assume 100 g of the compound is present then find the moles of each. Well take the mass of the hydrate subject it to PROLONGED drying and then measure the mass lost while drying. Click to see full answer.
 Source: chegg.com
Source: chegg.com
Calculate the moles of anhydrous CuSO4 present in a sample with 05 g CuSO4 at a. Divide the mass of your anhydrous heated salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. Use the molar mass of the compound to convert this to moles. Enter a chemical formula to calculate its molar mass and elemental composition. 2 Divide the smallest number into the others.
 Source: khanacademy.org
Source: khanacademy.org
Subtract the Constant Weight of the Empty Crucible from the Constant Weight After Heating 19. KAlSO42 36g -24g 12g Final weighing - mass of pon ass of anhydrous salt 34g-248 10g Step 2. The formula mass of the water is 4505g. In chemical formula you may use. I performed that same operation to find the number of moles of water lost using a molar mass of 1801528 gmol for H 2 O.
 Source: slideplayer.com
Source: slideplayer.com
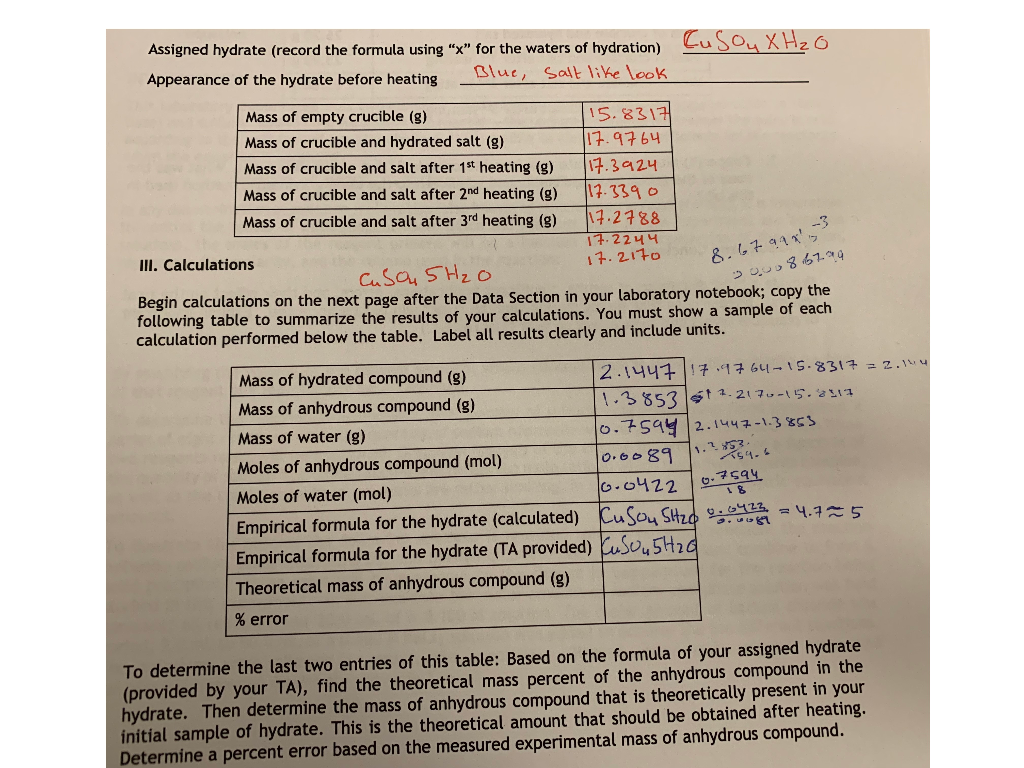
Answered 2 years ago Author has 17K answers and 7M answer views. Salt and can be removed upon heating yielding the anhydrous salt salt without water. Lastly to find the number of moles of water per mole of hydrate I divided the number of moles of anhydrous salt remaining by itself and by the number of moles of water lost. Zn — 23654 03517 S — 1132 034375 O — 2216 1375 H 2 O — 44 18 2444. Subtract the Constant Weight of the Empty Crucible from the Constant Weight After Heating 19.
 Source: in.pinterest.com
Source: in.pinterest.com
In order to determine the formula of the hydrate Anhydrous SolidxH2O the number of moles of water per mole of anhydrous solid x will be calculated by dividing the number of moles of water by the number of moles of the anhydrous solid Equation 212. 5 Mass of hydrated salt mass of anhydrous salt mass of water 320 g 1187 g 2013 g H 2 O. Lastly to find the number of moles of water per mole of hydrate I divided the number of moles of anhydrous salt remaining by itself and by the number of moles of water lost. The moles of salt should be the smaller of the two values. Answered 2 years ago Author has 17K answers and 7M answer views.

55 860 Views. This is how many moles of anhydrous sodium carbonate dissolved. Now the difference between the mass of the hydrate and the mass of the anhydrous salt will be equal to the mass of water of hydration. H 25 101 2 505g. 44 Votes Divide the mass of anhydrate by the molar mass of anhydrate to get moles of anhydrate.
 Source: chegg.com
Source: chegg.com
Divide the mass of your anhydrous heated salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. H 25 101 2 505g. KAlSO42 36g -24g 12g Final weighing - mass of pon ass of anhydrous salt 34g-248 10g Step 2. Capitalize the first letter in chemical symbol and use lower case for the remaining letters. The attempt at a solution.
 Source: chegg.com
Source: chegg.com
The moles of salt should be the smaller of the two values. 2 Divide the smallest number into the others. Can be calculated from the ratio of moles of anhydrous salt to moles of water. Grams of the dry salt divided by the molar mass moles B Calculate. Molar mass of MgSO4anhydrous is 1203676 gmol Convert between MgSO4anhydrous weight and moles.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find moles of anhydrous compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






