Your How to find moles of a compound in an equation images are available. How to find moles of a compound in an equation are a topic that is being searched for and liked by netizens now. You can Download the How to find moles of a compound in an equation files here. Find and Download all free images.
If you’re looking for how to find moles of a compound in an equation images information related to the how to find moles of a compound in an equation keyword, you have visit the ideal site. Our website frequently gives you hints for seeking the highest quality video and picture content, please kindly search and locate more enlightening video articles and graphics that match your interests.
How To Find Moles Of A Compound In An Equation. A mass of 2 moles of iron number of moles molar mass 2 56 112 g. To convert between grams and moles you would use the substances molar mass. Identify the limiting reagent in this reactant and the quantity of excess reagent in ml. Mass of one mole MnO2 8694g.
 How To Calculate The Number Of Moles From surfguppy.com
How To Calculate The Number Of Moles From surfguppy.com
25000 g of Cl2 are used and there are 70506 gmol of Cl2. Number of moles 95 8694. NaCl 229898 gL 354530 gL. For example suppose we have the balanced chemical equation. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl. Find the gfw of each compound do not combine them.
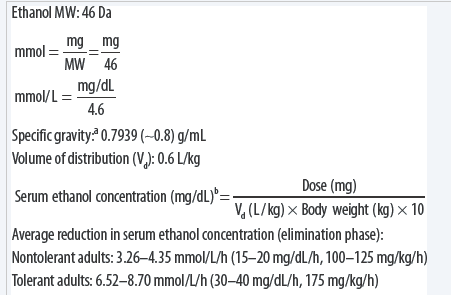
Finally divide the number of grams of the compound by the molar mass of the compound to find the number of moles.
Number of moles 1092 mol. A if the calculated moles needed is greater than the moles have for a given reactant then that reactant is the limiting reagent. A Calculate moles Mg massMg molar massMg molesMg m 2431 b Use the balanced chemical equation to determine the mole ratio O 2Mg molesO 2. NaCl Na Cl. Number of moles 95 8694. It allows you to easily convert between grams and moles of a.
 Source: youtube.com
Source: youtube.com
Find the gfw of each compound do not combine them. Number of moles 1092 mol. A balanced equation for the above reaction is written as follows. The molar mass is the amount in grams g of one mole of a compound. Number of moles formula is.
 Source: youtube.com
Source: youtube.com
B mass of 025 mole of iron number of moles molar mass. Notice in the chemical equation that we need to use 2 molecules of oxygen. So we multiply it by 2 and get 64. A if the calculated moles needed is greater than the moles have for a given reactant then that reactant is the limiting reagent. For example suppose we have the balanced chemical equation.
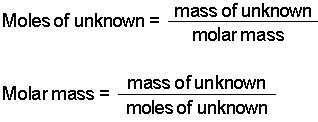 Source: chem.purdue.edu
Source: chem.purdue.edu
Fe 56 Solution. Use the periodic table to determine the atomic mass of each element in the molecule. It allows you to easily convert between grams and moles of a. Therefore NaCl 584538 gL. Grams of mass divided by molecular weight gives you moles of molecules.
 Source: youtube.com
Source: youtube.com
So lets say you figured you got about 02 moles of chemical bicycles in your pile. Mass of one mole MnO2 8694g. From the equation 2 mol of. M r of NaOH 23 16 1 40. To convert grams to moles start by multiplying the number of atoms by the atomic weight for each element in the compound.
 Source: surfguppy.com
Source: surfguppy.com
Use the periodic table to determine the atomic mass of each element in the molecule. 500 22990 21749. Determine the molecular formula of the molecule. Identify the limiting reagent in this reactant and the quantity of excess reagent in ml. Divide the number of grams of each reactant by the number of grams per mole for that reactant.
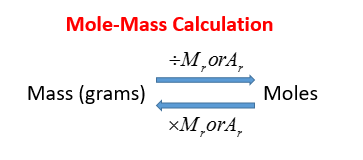 Source: onlinemathlearning.com
Source: onlinemathlearning.com
Then add all of your answers together to find the molar mass of the compound. Mass of MnO2 95g. Number of moles 95 8694. Identify the limiting reagent in this reactant and the quantity of excess reagent in ml. A balanced equation for the above reaction is written as follows.
 Source: youtube.com
Source: youtube.com
If your compound were potassium sulfide K₂S you would add the mass of 2 mol of potassium 2 39097 g and the mass of 1 mol of sulfur 32064 g. To convert grams to moles start by multiplying the number of atoms by the atomic weight for each element in the compound. This would be the mass of 1 mol of K₂S. Find the relation between moles of N 2 H 4 and N 2 by using the coefficients of the balanced equation. NaCl Na Cl.
 Source: dummies.com
Source: dummies.com
Fe 56 Solution. To convert between grams and moles you would use the substances molar mass. 25000 g of Cl2 are used and there are 70506 gmol of Cl2. Find the gfw of each compound do not combine them. Number of moles Mass of substance Mass of one mole.
 Source: nobel.scas.bcit.ca
Source: nobel.scas.bcit.ca
A mass of 2 moles of iron number of moles molar mass 2 56 112 g. This would be the mass of 1 mol of K₂S. NaCl 229898 gL 354530 gL. 2Al 3Cl 2 2AlCl 3. Therefore NaCl 584538 gL.
 Source: youtube.com
Source: youtube.com
Multiply each elements atomic mass by the number of atoms of that element in the molecule. NaCl 229898 gL 354530 gL. Find the gfw of each compound do not combine them. 2 mol N 2 H 4 is proportional to 3 mol N 2 In this case we want to go from moles of N 2 H 4 to moles of N 2 so the conversion factor is 3 mol N 2 2 mol N 2 H 4. Find the relation between moles of N 2 H 4 and N 2 by using the coefficients of the balanced equation.
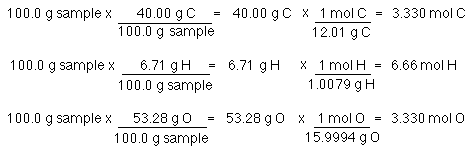 Source: westfield.ma.edu
Source: westfield.ma.edu
This makes 1 mole of carbon dioxide and 2 moles of water. Or pounds of bikes in the pile divided by the weight of each bike gives you the number of bikes. Use the periodic table to determine the atomic mass of each element in the molecule. Then add all of your answers together to find the molar mass of the compound. A balanced equation for the above reaction is written as follows.
 Source: chem.fsu.edu
Source: chem.fsu.edu
Determine the molecular formula of the molecule. Its easy to find the molecular mass of a compound with these steps. If your compound were potassium sulfide K₂S you would add the mass of 2 mol of potassium 2 39097 g and the mass of 1 mol of sulfur 32064 g. From the equation 2 mol of. To convert grams to moles start by multiplying the number of atoms by the atomic weight for each element in the compound.
 Source: centraltutors.co.uk
Source: centraltutors.co.uk
To convert grams to moles start by multiplying the number of atoms by the atomic weight for each element in the compound. 21749 moles of Na are used in this reaction. M r of NaOH 23 16 1 40. Notice in the chemical equation that we need to use 2 molecules of oxygen. Where we start with a given number of moles of a substance and calculate the mass of another substance involved in the chemical equation or vice versa.
 Source: khanacademy.org
Source: khanacademy.org
So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl. 2 c Use the mole ratio to calculate moles O 2. Divide the number of grams of each reactant by the number of grams per mole for that reactant. A balanced equation for the above reaction is written as follows. Number of moles 95 8694.
 Source: slideplayer.com
Source: slideplayer.com
B mass of 025 mole of iron number of moles molar mass. Find the gfw of each compound do not combine them. Number of moles 1092 mol. So lets say you figured you got about 02 moles of chemical bicycles in your pile. Mass of one mole MnO2 8694g.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find moles of a compound in an equation by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.