Your How to find moles of a compound in a solution images are ready. How to find moles of a compound in a solution are a topic that is being searched for and liked by netizens now. You can Get the How to find moles of a compound in a solution files here. Find and Download all free photos.
If you’re looking for how to find moles of a compound in a solution pictures information linked to the how to find moles of a compound in a solution keyword, you have pay a visit to the ideal blog. Our site frequently gives you suggestions for downloading the highest quality video and image content, please kindly hunt and locate more informative video content and graphics that match your interests.
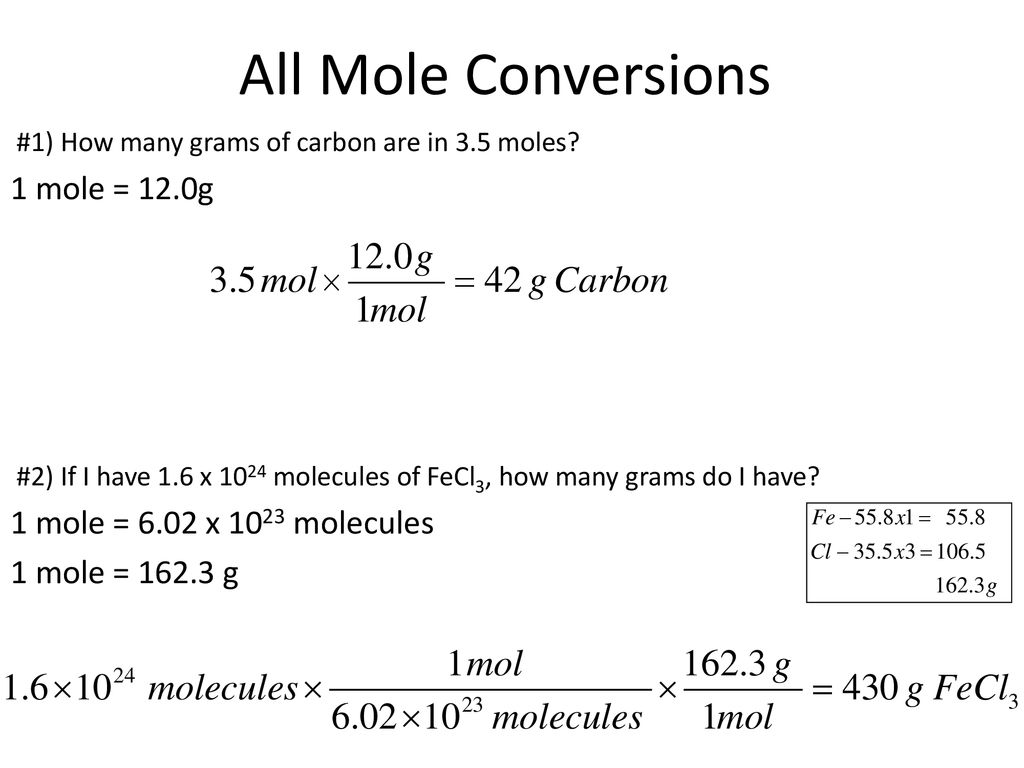
How To Find Moles Of A Compound In A Solution. How do you calculate the neutralization of a mole. To compute any of the elements in the molarity equation you need to input the other three and choose the desired measurement unit. Now you need to find the moles to complete the problem. Determine MW using a periodic table by adding the atomic mass of each atom in the chemical formula.
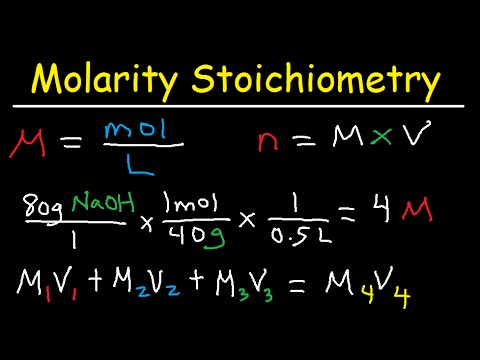 Molarity Dilution Problems Solution Stoichiometry Grams Moles Liters Volume Calculations Chemistry Youtube From youtube.com
Molarity Dilution Problems Solution Stoichiometry Grams Moles Liters Volume Calculations Chemistry Youtube From youtube.com
How do you calculate molarity. This is a great chemistry tool for those who need to determine a solutions molar concentration from the mass volume and molecular weight. Third find how many molecules of Carbon. In many older books or articles you can find different units of molar solutions - moles per liter moll. O - 3 moles of O for 1 mole of KNO3. How do you find the molar mass of an unknown compound.
How do you calculate the neutralization of a mole.
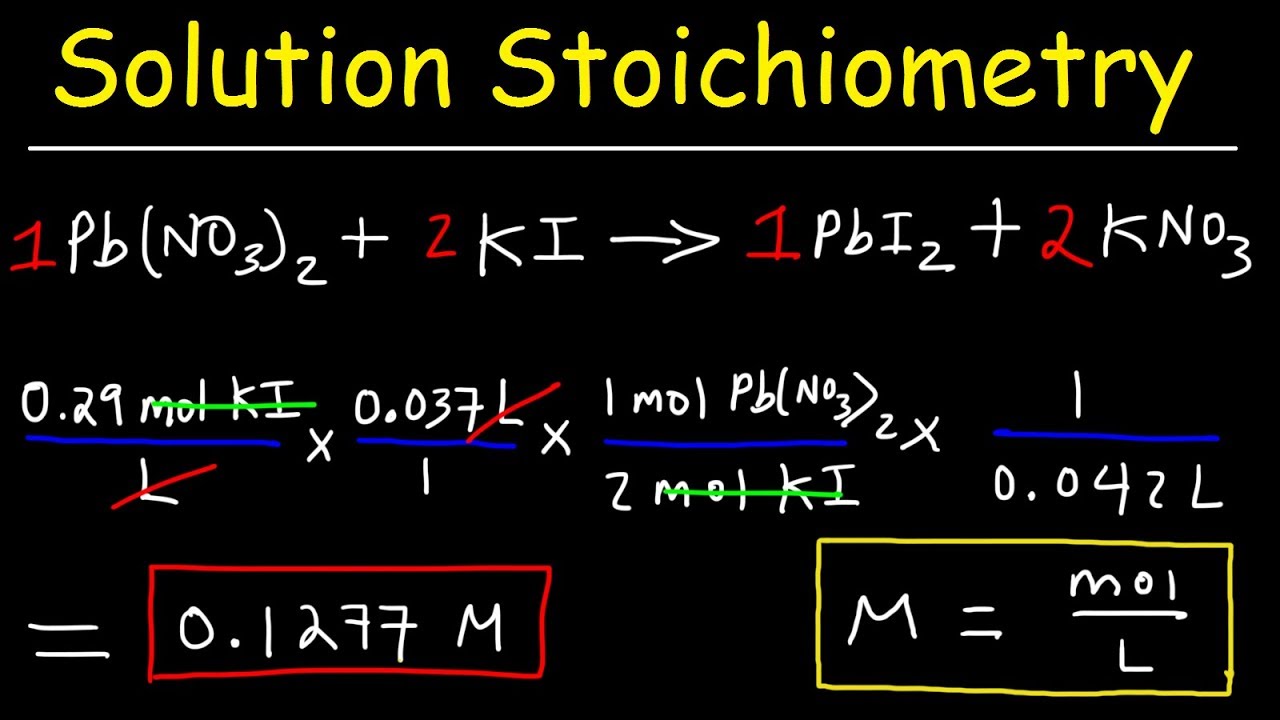
In our example 05 moles of water 01 moles copper sulfate 51 ratio. Dissolving a known amount of a reactant in a known amount of. M of Cl- M of CuCl 2 x ionsolute M of Cl- 012 moles CuCl 2 L x 2 moles of Cl-1 mole CuCl 2 M of Cl- 024 moles of Cl-L M of Cl- 024 M Answer The molarity of the Cl ions in the solution is 024 M. How do you calculate the neutralization of a mole. O - 3 moles of O for 1 mole of KNO3. Beside this how do you calculate KSP.
 Source: pinterest.com
Source: pinterest.com
M of Cl- M of CuCl 2 x ionsolute M of Cl- 012 moles CuCl 2 L x 2 moles of Cl-1 mole CuCl 2 M of Cl- 024 moles of Cl-L M of Cl- 024 M Answer The molarity of the Cl ions in the solution is 024 M. 16431022 x 2 32871022 molecules of C. Using mole ratios the Ag will go up by 2 x 131 x 10-4 molesL 262 x 10-4 molesL. Now you need to find the moles to complete the problem. NaCl 1 Na 1 Cl 1 2299 gmol 1 3545 gmol 5844 g mol We have determined our molar mass M and calculated n in Step 1.
 Source: pinterest.com
Source: pinterest.com
Then use the molality equation to calculate the moles of solute. How do you calculate the neutralization of a mole. How do you find the molar mass of an unknown compound. Determine the change in boiling or freezing point temperature solution and pure solvent. Scroll down the page for more examples and solutions.
 Source: pinterest.com
Source: pinterest.com
Determine MW using a periodic table by adding the atomic mass of each atom in the chemical formula. A homogeneous liquid solution is often the preferred medium to handle various quantities of ionic compounds and carry out chemical reactions. First find the number of mols in entire compound C2H6O. 16431022 x 2 32871022 molecules of C. To compute any of the elements in the molarity equation you need to input the other three and choose the desired measurement unit.
 Source: pinterest.com
Source: pinterest.com
The solubility of Ag2CrO4 in water is 131 x 10-4 molesL. For finding out this you have to multiply the mass of solute by its molar. N - 00495 mol. Lets do an example. It refers to the number of moles per litre of solution.
 Source: pinterest.com
Source: pinterest.com
1257g C2H6O x 1 mol C2H6O 46068g 002729 mol C2H6O. Third find how many molecules of Carbon. Molarity moles of solute litres of solution. To compute any of the elements in the molarity equation you need to input the other three and choose the desired measurement unit. First find the number of mols in entire compound C2H6O.
 Source: khanacademy.org
Source: khanacademy.org
02729 mol C2H6O x 60221023 1 mol 16431022 molecules. In this case n 5 1 101 96 0 05002 m o l. Scroll down the page for more examples and solutions. By rearranging the molarity formula where molarity equals moles of solute divided by liters of solution the amount of moles. A homogeneous liquid solution is often the preferred medium to handle various quantities of ionic compounds and carry out chemical reactions.
 Source: youtube.com
Source: youtube.com
Molecular weight MW is the weight of one mole of a chemical. M of Cl- M of CuCl 2 x ionsolute M of Cl- 012 moles CuCl 2 L x 2 moles of Cl-1 mole CuCl 2 M of Cl- 024 moles of Cl-L M of Cl- 024 M Answer The molarity of the Cl ions in the solution is 024 M. It is one of the ways to measure the concentration of an element ion or compound in solution. The formula for molarity the molarity equation is M n v. The molarity M of a solution is the number of moles of solute dissolved in one liter of solution.
 Source: chem.fsu.edu
Source: chem.fsu.edu
Remember that one cubic decimeter equals to one liter so these two notations express the same numeric values. How to Calculate Molarity for a Solution Watch later Watch on Answer link. This means that for every unit of CuSO4 present we have 5 molecules of water. Molecular weight MW is the weight of one mole of a chemical. Divide the number of moles by the number of liters.
 Source: hu.pinterest.com
Source: hu.pinterest.com
Sep 06 2020 The formula for the amount of an element in a compound is. N the number of moles of a compound. The solubility of Ag2CrO4 in water is 131 x 10-4 molesL. Calculate the number of moles of OH-. To calculate the molarity of a solution you divide the moles of solute by the volume of the solution expressed in liters.
 Source: youtube.com
Source: youtube.com
N - 00495 mol. How do you find the molar mass of an unknown compound. Now that you have the number of liters you can divide the number of moles of solute by this value in order to find the molarity. If youre dealing with molarity the number of moles of a solute can be determined by n C V solution - with the volume andor the molarity usually given. Calculate the number of moles of OH-.
 Source: youtube.com
Source: youtube.com
Besides for calculating molarity we use the following equation. To calculate the molality of the solution. Dissolving a known amount of a reactant in a known amount of. If youre dealing with molarity the number of moles of a solute can be determined by n C V solution - with the volume andor the molarity usually given. 02729 mol C2H6O x 60221023 1 mol 16431022 molecules.
 Source: youtube.com
Source: youtube.com
Now that you have the number of liters you can divide the number of moles of solute by this value in order to find the molarity. Moles of element n moles elementone mole of compound K - 1 mole of K for 1 mole of KNO3. There are four components in the equation therefore there are four fields in the molarity calculator. O - 3 moles of O for 1 mole of KNO3. 1257g C2H6O x 1 mol C2H6O 46068g 002729 mol C2H6O.
 Source: pinterest.com
Source: pinterest.com
In this case n 5 1 101 96 0 05002 m o l. Determine the change in boiling or freezing point temperature solution and pure solvent. Mass moles molarity and volume. Second find how many molecules of each element are in it. Mass percent grams of solute grams of solution x 100.
 Source: pinterest.com
Source: pinterest.com
To calculate the molality of the solution. How do you calculate molarity. Third find how many molecules of Carbon. By rearranging the molarity formula where molarity equals moles of solute divided by liters of solution the amount of moles. How do you calculate the neutralization of a mole.
 Source: pinterest.com
Source: pinterest.com
1257g C2H6O x 1 mol C2H6O 46068g 002729 mol C2H6O. Beside this how do you calculate KSP. Molecular weight MW is the weight of one mole of a chemical. Besides for calculating molarity we use the following equation. Find the molarity of the solute.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to find moles of a compound in a solution by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.