Your How to find moles of a compound from grams images are available in this site. How to find moles of a compound from grams are a topic that is being searched for and liked by netizens now. You can Download the How to find moles of a compound from grams files here. Get all royalty-free images.
If you’re looking for how to find moles of a compound from grams pictures information linked to the how to find moles of a compound from grams keyword, you have visit the ideal blog. Our website always gives you hints for seeking the highest quality video and image content, please kindly hunt and find more enlightening video content and graphics that match your interests.
How To Find Moles Of A Compound From Grams. Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4. To go from moles to grams multiply by the formula mass. First determine the molar masses of the compounds component elements. Click to see full answer.
 How To Convert Grams To Moles Very Easy Youtube From youtube.com
How To Convert Grams To Moles Very Easy Youtube From youtube.com
Multiply the variety of moles by the molar mass to acquire the ultimate reply in grams. One mole equals to a very large number of particles. Multiply the number of moles by the molar mass to obtain the final answer in grams. Look for the atomic masses of hydrogen sulfur and oxygen. Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4. This is the molar mass of the compound.
NaCl 229898 gL 354530 gL.
Finding the Molar Mass of a Compound. NaCl Na Cl. Multiply the variety of moles by the molar mass to acquire the ultimate reply in grams. Finding the Molar Mass of a Compound. For example if you have 170 mol of NaCl then. If it isnt then it may be discovered by wanting on the chemical.
 Source: study.com
Source: study.com
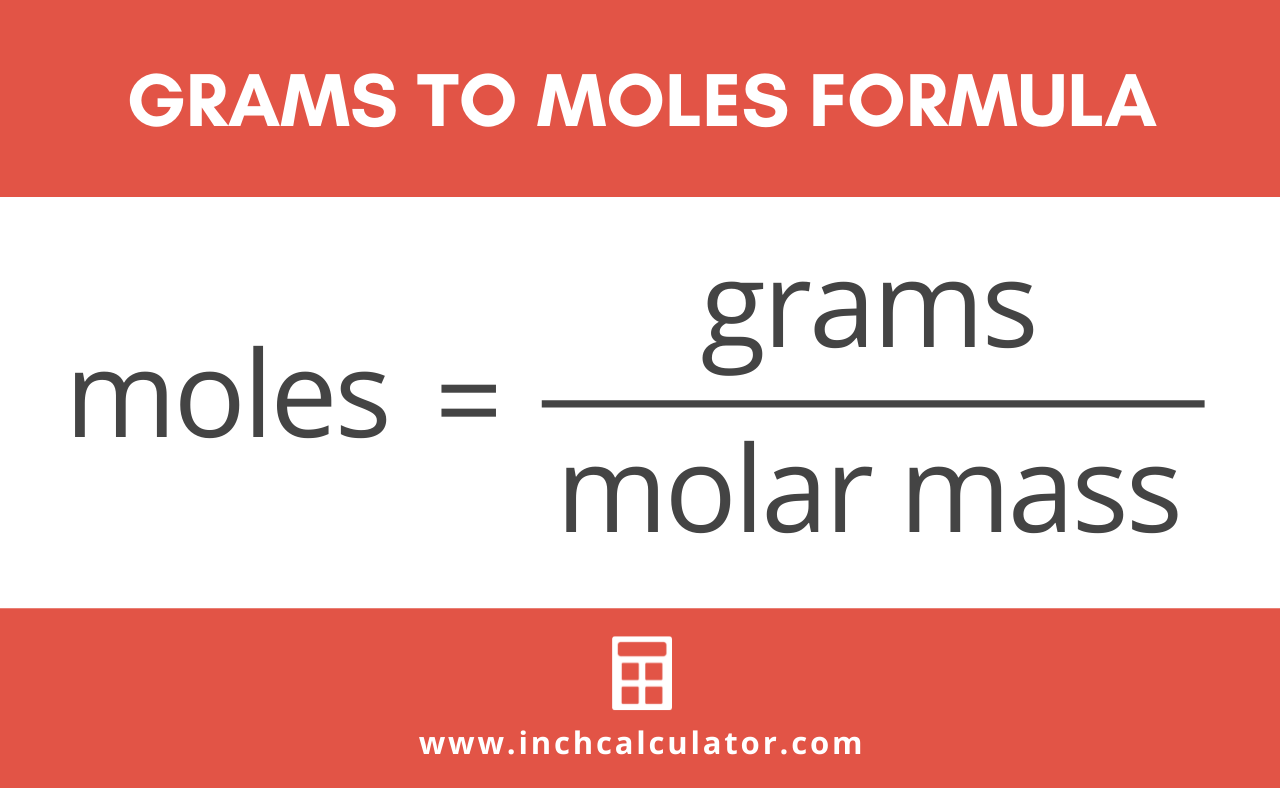
The formula for moles to grams is given by. Since you need to find for 360 mol of H. One mole of a compound contains Avogadros number 6022 x 10 23 of molecules molecular compound or formula units ionic compoundThe molar mass of a compound tells you the mass of 1 mole of that substance. To find how many grams are in a mole of a compound involves extra steps. 1 gram of mole is the amount of the molecules used to represent the moles of the molecules that are present in one mole of the substance.
 Source: inchcalculator.com
Source: inchcalculator.com
Divide the Quantity of the Compound in Grams by the Molecular Weight We will now convert 100g of NaOH to moles. One mole equals to a very large number of particles. 170 mol 58443 g 1 mol 994 g. The unit is typically gmol. To go from moles to grams multiply by the formula mass.
 Source: youtube.com
Source: youtube.com
Divide the number of grams of the compound by its molecular mass. Therefore NaCl 584538 gL. Find the molecular mass of the compound. Whereas units such as grams or pounds describe the mass of a chemical moles describe the number of particles – either atoms or molecules – of that compound. 602 x 1023 of them.
 Source: chem.fsu.edu
Source: chem.fsu.edu
Hence one mole of H 2 SO 4 weights 106076 grams. The unit is often gmol. 170 mol 58443 g 1 mol 994 g. If you are given the molarity of any solution and asked to calculate the number of moles simply multiply the molarity with the total volume of the solution in litres. To go from grams to moles divide the grams by the molar mass.
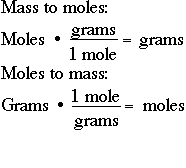 Source: socratic.org
Source: socratic.org
It has units of grams per mole. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. The answer is the number of moles of that mass of compound. Divide the mass of the compound in grams by the molar mass you just calculated. 3 Following step three we obtain.
 Source: study.com
Source: study.com
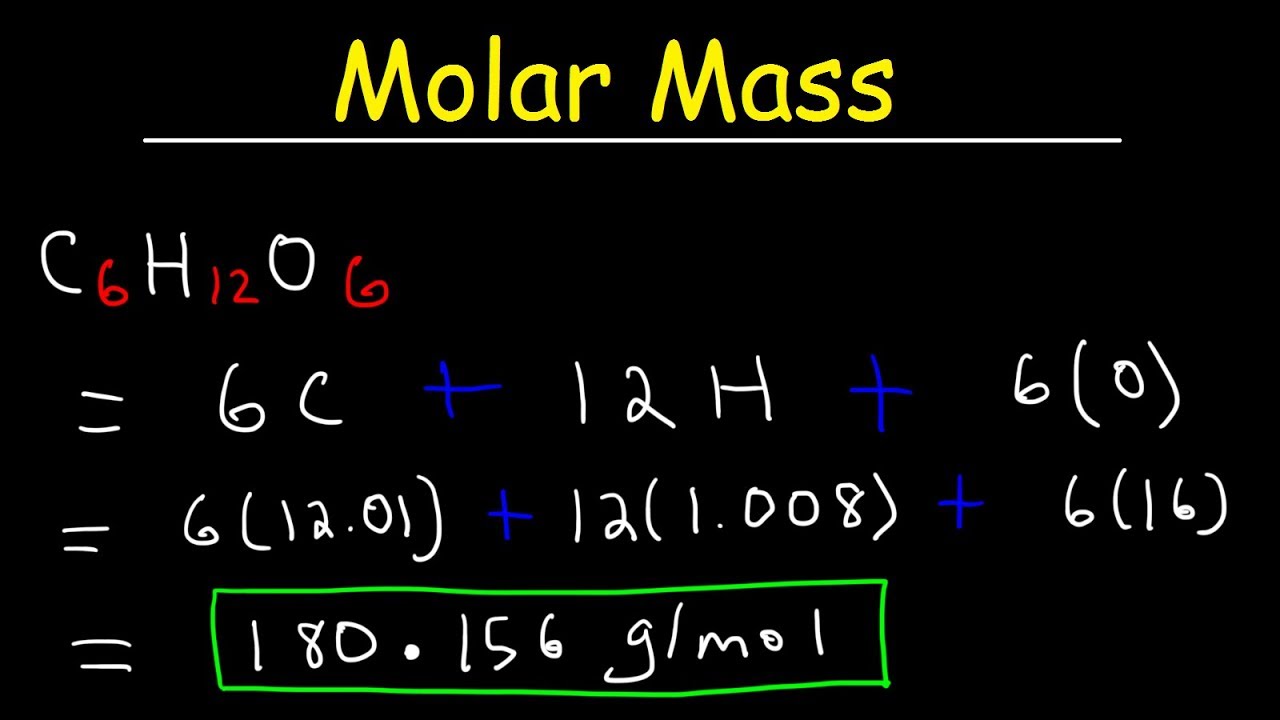
In order to determine the number of moles of a given compound the first thing you need to do is find the molecular mass or molecular weight of the compound in question. It is important for proper cancelling of units that you remember to write this unit down when using a molar mass. NaCl Na Cl. 250 moles x 122550 gmole 306375 grams. 3 Following step three we obtain.
 Source: youtube.com
Source: youtube.com
Therefore the molecular mass of H 2 SO 4 is. Finding the Molar Mass of a Compound. Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4. For example if you have 170 mol of NaCl then. Multiply the number of moles by the molar mass to obtain the final answer in grams.
 Source: slideplayer.com
Source: slideplayer.com
The unit is typically gmol. Shows how to use molar conversions to convert from grams to moles and moles to grams. TextMolar Mass 107868 for calculations tap Molar Mass Calculator By using moles to grams formula. Multiply the number of moles by the molar mass to obtain the final answer in grams. In order to determine the number of moles of a given compound the first thing you need to do is find the molecular mass or molecular weight of the compound in question.
 Source: youtube.com
Source: youtube.com
You can find the moles of any mass of any compound. Multiply the number of moles by the molar mass to obtain the final answer in grams. N m M where M is the molar mass of this material. To go from moles to grams multiply by the formula mass. 602 x 1023 of them.
 Source: surfguppy.com
Source: surfguppy.com
The unit is often gmol. Divide the variety of grams of the compound by its molecular mass. Find the molecular mass of the compound. Therefore the molecular mass of H 2 SO 4 is. Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4.
 Source: slideplayer.com
Source: slideplayer.com
To convert grams to moles start by multiplying the number of atoms by the atomic weight for each element in the compound. Therefore NaCl 584538 gL. You can see a listing of all my videos at my website httpwwwstepby. Divide the number of grams of the compound by its molecular mass. To go from moles to grams multiply by the formula mass.
 Source: youtube.com
Source: youtube.com
This is the molar mass of the compound. Now we have to perform moles to grams calculation. The number of moles present in a compound is often given to the student in the problem. To convert moles into grams determine the number of moles preset and the molar mass of the compound. Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom.
 Source: wikihow.com
Source: wikihow.com
So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl. Molar mass of Okay 391 g. 170 mol 58443 g 1 mol 994 g. Multiply the number of moles by the molar mass to obtain the final answer in grams. To go from moles to grams multiply by the formula mass.
 Source: khanacademy.org
Source: khanacademy.org
Moles to Grams Conversion Formulation. The number of moles present in a compound is often given to the student in the problem. First determine the molar masses of the compounds component elements. Molar mass of Okay 391 g. N m M where M is the molar mass of this material.
 Source: study.com
Source: study.com
Whereas units such as grams or pounds describe the mass of a chemical moles describe the number of particles – either atoms or molecules – of that compound. If it is not then it can be found by looking at the. Multiply the variety of moles by the molar mass to acquire the ultimate reply in grams. To go from moles to grams multiply by the formula mass. Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find moles of a compound from grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






