Your How to find moles given grams and volume images are available in this site. How to find moles given grams and volume are a topic that is being searched for and liked by netizens now. You can Get the How to find moles given grams and volume files here. Find and Download all royalty-free vectors.
If you’re looking for how to find moles given grams and volume pictures information related to the how to find moles given grams and volume keyword, you have come to the ideal site. Our website frequently gives you hints for viewing the maximum quality video and image content, please kindly surf and find more enlightening video articles and images that match your interests.
How To Find Moles Given Grams And Volume. Of moles of soluteliter of solution no. Lets see how this works. G dm3 Mass of solute in grams Vol. The equation for this half-reaction is.
 Calculating Molar Mass And Number Of Moles Worked Example Video Khan Academy From khanacademy.org
Calculating Molar Mass And Number Of Moles Worked Example Video Khan Academy From khanacademy.org
Lets see how this works. Calculate the number of moles of solute. Once we get the value for moles we can then divide the mass of gas by moles to get the molar mass. Please refer to the lessons about calculating the molecular weight and molar mass of a substance if you are not sure how to calculate a molar mass. A Volume of CO 2 number of moles of CO 2 224 L 5 224. Of moles of I2 mass in gramsmolar mass 46525381 001832 mol M 001832 mol0235 L 00780 molL What is the ornaments can be.
4 e- 4 H 2 Ol 2 H 2 g 4 OH-aq Calculate the number of moles of electrons.
From you calculate the molar mass to be 1021 gmol. Hence molecular mass of Fe Atomic mass of Fe 56 g. Using the ideal gas law to calculate a change in volume. NaCl 229898 gL 354530 gL. Of moles of soluteliter of solution no. 1 FW of Na 2HPO 4.
 Source: pinterest.com
Source: pinterest.com
Using the ideal gas law to calculate a change in volume. Remember at STP 1 mole of any gas occupies 224 L Write the equation for the half-reaction that takes place. 0700 moles are given in the problem. Divide the mass by the molar mass to get the number of moles. Hydrogen is produced during the reduction of water at the cathode.
 Source: youtube.com
Source: youtube.com
Convert grams to moles. NaCl Na Cl. A mole is defined as the number of substances containing particles of that substance as many as the atoms contained in 12000 grams of carbon atoms 12. Once we get the value for moles we can then divide the mass of gas by moles to get the molar mass. Mass 56 Given mass of Fe 785 g.
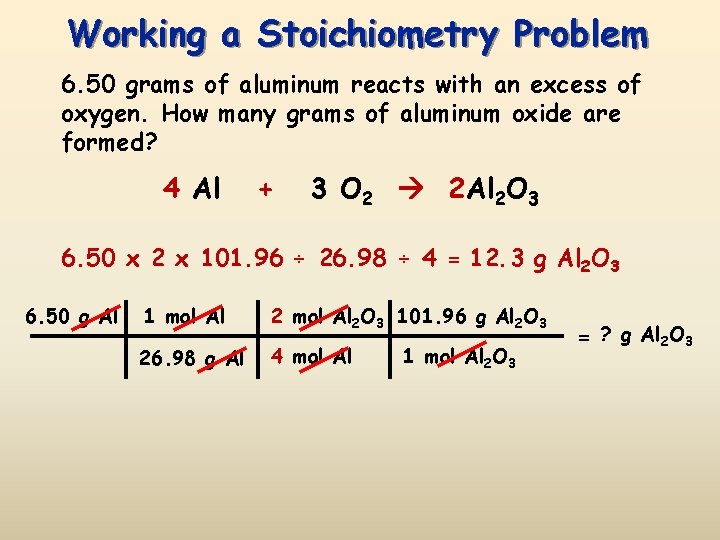 Source: slidetodoc.com
Source: slidetodoc.com
You can rearrange this equation to solve the problem that is given in the question. Therefore NaCl 584538 gL. Molar Concentration to Mass Concentration conversion Formula to calculate pH from molarity. Using the ideal gas law to calculate number of moles. 0700 moles are given in the problem.
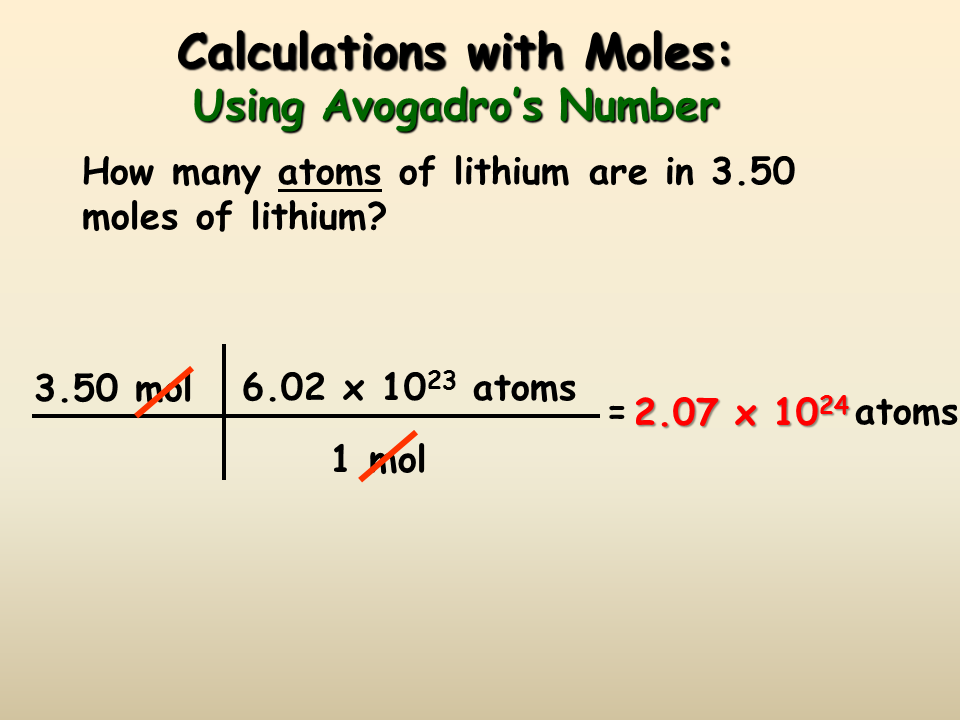
Molarity no. Mass 56 Given mass of Fe 785 g. Gas volumes from moles and grams. Convert millilitres to grams. From you calculate the molar mass to be 1021 gmol.
 Source: pinterest.com
Source: pinterest.com
A mole is defined as the number of substances containing particles of that substance as many as the atoms contained in 12000 grams of carbon atoms 12. If we know mass pressure volume and temperature of a gas we can calculate its molar mass by using the ideal gas equation. Molarity no. 1 FW of Na 2HPO 4. The molar mass of H 2 O 2 is 340146 gramsmole.
 Source: youtube.com
Source: youtube.com
Example If you want to find the molar mass of common salt Sodium Chloride- NaCl You add the mass of each element of it. Recall that the ideal gas equation is given as. Divide the mass by the molar mass to get the number of moles. Convert millilitres to grams. You have to work out the molar mass of CHO.
 Source: wikihow.com
Source: wikihow.com
Answer 1 of 11. Calculate how many moles are mentioned in the question. Calculate the molar mass of the solute. Mass 56 Given mass of Fe 785 g. Therefore NaCl 584538 gL.
 Source: youtube.com
Source: youtube.com
You need to know the molar mass of the substance. You have to work out the molar mass of CHO. To Calculate Number of Moles. 0700 moles are given in the problem. Using the ideal gas law to calculate a change in volume.
 Source: youtube.com
Source: youtube.com
785 g of Fe at. Hence molecular mass of Fe Atomic mass of Fe 56 g. Using the ideal gas law to calculate a change in volume. You can rearrange this equation to solve the problem that is given in the question. Remember at STP 1 mole of any gas occupies 224 L Write the equation for the half-reaction that takes place.
 Source: pinterest.com
Source: pinterest.com
Mass 56 Given mass of Fe 785 g. Solution Since the molar amount of solute and the volume of solution are. Number of moles of Fe. One calcium 4008 gramsmole Two nitrogen 14012 2802. One mole consists of Avogadro number of atoms.
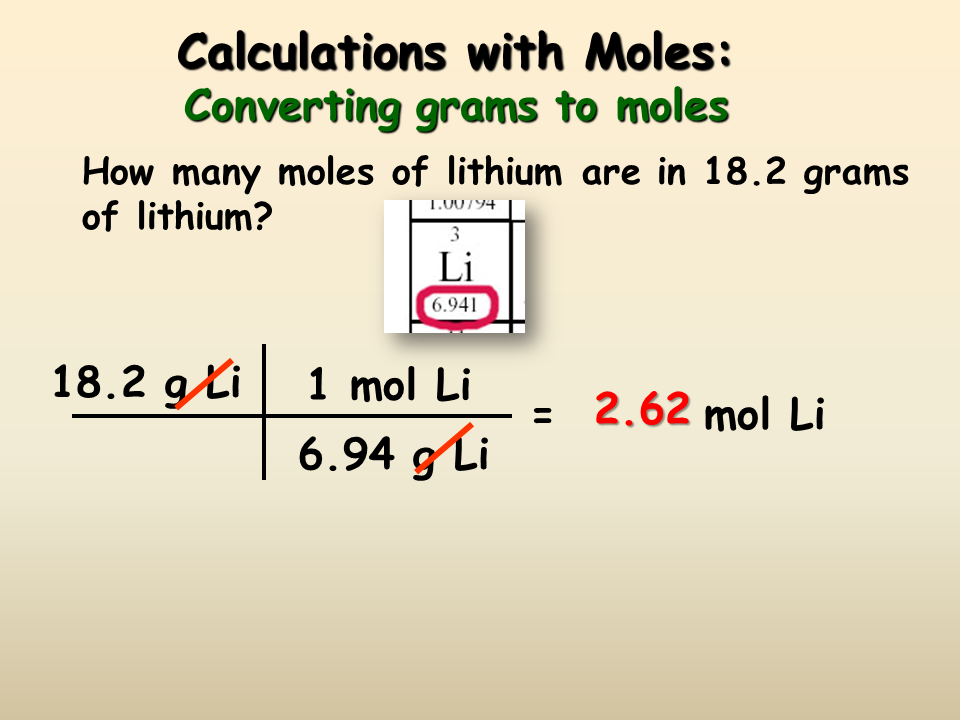
The molar mass of H 2 O 2 is 340146 gramsmole. There are two steps. If you know the quantity of mole it can be converted into grams and vice versa. Ca NO_3_2 times frac 1. In these lessons we will learn the Molar Volume Avogadros Law how to calculate gas volumes given moles and grams how to calculate moles given gas volumes and how to calculate gas volumes given the chemical equation.
 Source: khanacademy.org
Source: khanacademy.org
G dm3 Mass of solute in grams Vol. Convert grams to moles. You have to work out the molar mass of CHO. Molar Concentration to Mass Concentration conversion Formula to calculate pH from molarity. How to convert a volume into an amount in moles.
 Source: pinterest.com
Source: pinterest.com
You need to know the molar mass of the substance. Example If you want to find the molar mass of common salt Sodium Chloride- NaCl You add the mass of each element of it. The molar volume is the volume occupied by one mole of a substance chemical element or chemical compound at a given. How to convert a volume into an amount in moles. Gas volumes from moles and grams.
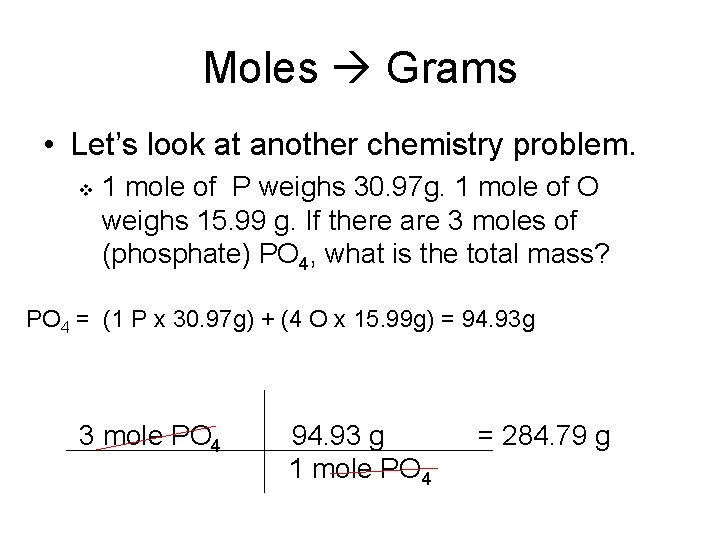 Source: slidetodoc.com
Source: slidetodoc.com
One mole consists of Avogadro number of atoms. Hence molecular mass of Fe Atomic mass of Fe 56 g. Recall that the ideal gas equation is given as. 0700 moles are given in the problem. The molar mass of H 2 O 2 is 340146 gramsmole.
 Source: youtube.com
Source: youtube.com
You can rearrange this equation to solve the problem that is given in the question. If we know mass pressure volume and temperature of a gas we can calculate its molar mass by using the ideal gas equation. You use the molar mass. The equation for this half-reaction is. You can rearrange this equation to solve the problem that is given in the question.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find moles given grams and volume by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






