Your How to find moles from grams and volume images are available in this site. How to find moles from grams and volume are a topic that is being searched for and liked by netizens now. You can Download the How to find moles from grams and volume files here. Get all free photos.
If you’re searching for how to find moles from grams and volume images information connected with to the how to find moles from grams and volume interest, you have visit the ideal blog. Our site frequently provides you with suggestions for viewing the highest quality video and image content, please kindly hunt and locate more enlightening video content and graphics that fit your interests.
How To Find Moles From Grams And Volume. Ca NO_3_2 times frac 1. After about a semester and a half I had forgotten how to convert from grams to molesThis article was extremely clear precise and definitive. Convert millilitres to grams. A mole is defined as the number of substances containing particles of that substance as many as the atoms contained in 12000 grams of carbon atoms 12.
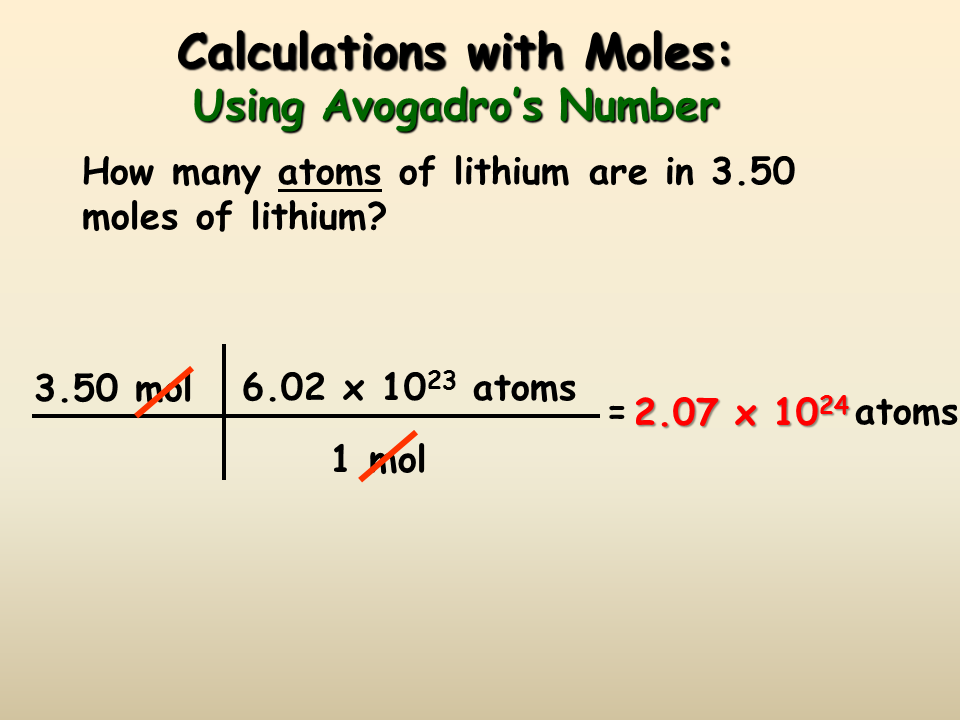
Volume 05 24 12 dm 3 Remember that 1 dm 3 1 000 cm 3 so the volume is also 12 000 cm 3 The equation can be rearranged to find the number of moles if. 461314x16 142 Molar concentration Mass conc. Calculate the molar mass of the solute. In the same way HCl has one atom of Hydrogen H and one atom of Chlorine Cl. How to Calculate moles based on molarity and volume. This is only possible with acids and bases.
You need the concentration of the solution to do this.
One calcium 4008 gramsmole Two nitrogen 14012 2802. How to convert an amount in moles to a volume in mL. Convert grams to moles. N m M where M is the molar mass of this material. In the same way HCl has one atom of Hydrogen H and one atom of Chlorine Cl. Answer 1 of 11.
 Source: youtube.com
Source: youtube.com
How to Calculate moles based on molarity and volume. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. Answer 1 of 11. One calcium 4008 gramsmole Two nitrogen 14012 2802. Gas volumes from moles and grams.
 Source: pinterest.com
Source: pinterest.com
Calculate the molar mass of the solute. In the same way HCl has one atom of Hydrogen H and one atom of Chlorine Cl. From compound to compound to a number of atoms in a molecule vary. From you calculate the molar mass to be 1021 gmol. Therefore NaCl 584538 gL.
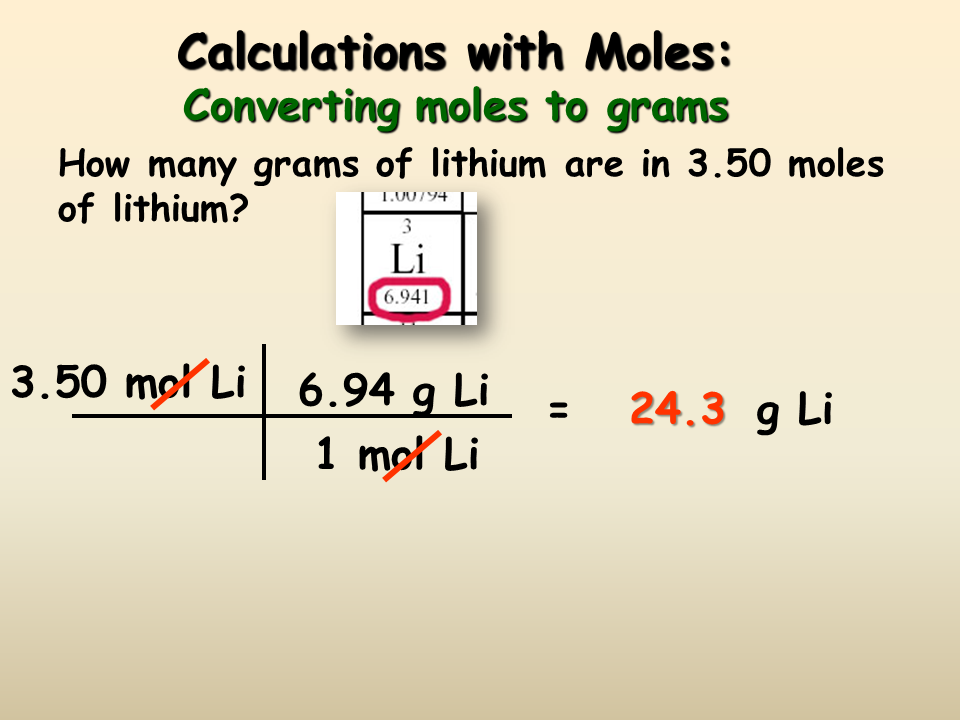
A gramg is a metric unit of mass and was formally defined as the absolute weight of a volume of pure water equal to the cube of the hundredth part of a meter and at the temperature of melting ice. Answer 1 of 11. You have to work out the molar mass of CHO. A gramg is a metric unit of mass and was formally defined as the absolute weight of a volume of pure water equal to the cube of the hundredth part of a meter and at the temperature of melting ice. You can rearrange this equation to solve the problem that is given in the question.
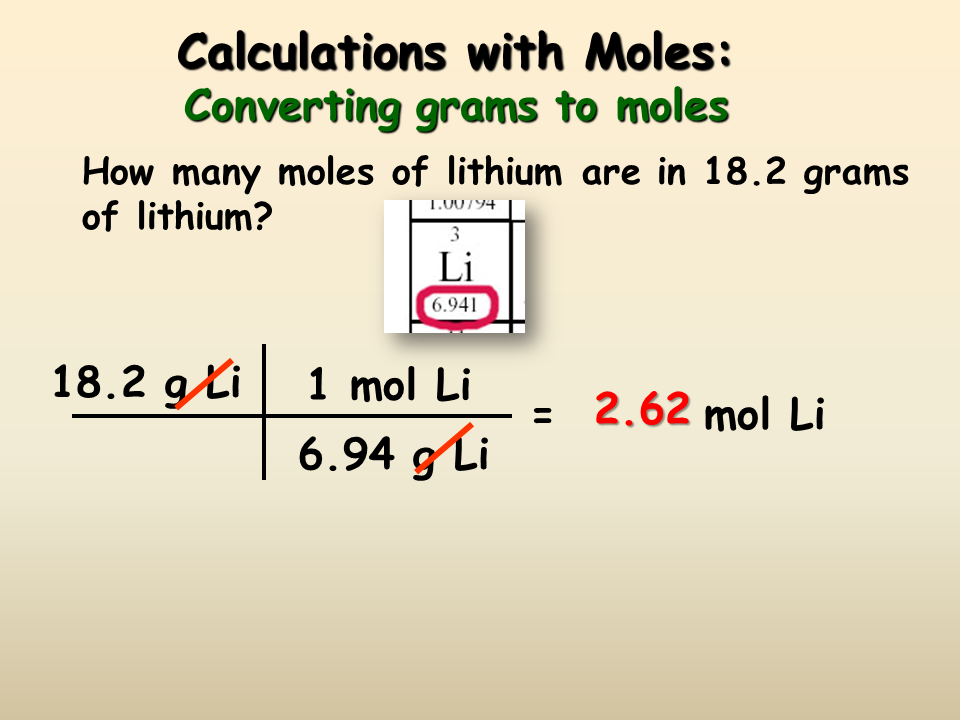
Therefore NaCl 584538 gL. How to Calculate moles based on molarity and volume. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. You can rearrange this equation to solve the problem that is given in the question. Calculate the volume of solution.

To convert moles to g we multiply the. Calculate the volume of carbon dioxide gas CO 2 occupied by a 5 moles and b 05 moles of the gas occupied at STP. For example one molecule of H2O has two atoms of Hydrogen H and one atom of Oxygen O. Solution Since the molar amount of solute and the volume of solution are. Since moles grams of compoundthe molar mass to find the mass you will need to do mass molar mass x molarity.
 Source: pinterest.com
Source: pinterest.com
108 g 1 mol1021 g 0106 mol. NaCl 229898 gL 354530 gL. Calculate the number of moles of solute. C 1 V 1 c 2 V 2 V 1 c 2 V 2 c 1 350 m o l l 1 750 m l 120 m o l l 1 21875 m l 219 m l. Therefore NaCl 584538 gL.
 Source: slideplayer.com
Source: slideplayer.com
108 g 1 mol1021 g 0106 mol. Calculate the volume of solution. Convert millilitres to grams. Set up Equation Density gL molar mass molar volume Density 838𝑔 𝑜 224𝐿 𝑜 or Density 838 g1 mol x 1 mol 224 L Density of Kr 374 gL. Calculate the molar mass of the solute.
 Source: pinterest.com
Source: pinterest.com
Once you have the molar mass multiply the number of grams of solute by 1 over the molar mass to convert the grams into moles. In one mole of a substance there are 6022 x 10 23 particles. This is only possible with acids and bases. The unit is typically gmol. Since moles grams of compoundthe molar mass to find the mass you will need to do mass molar mass x molarity.
 Source: youtube.com
Source: youtube.com
One calcium 4008 gramsmole Two nitrogen 14012 2802. Gas volumes from moles and grams. Calculate the volume of carbon dioxide gas CO 2 occupied by a 5 moles and b 05 moles of the gas occupied at STP. In one mole of a substance there are 6022 x 10 23 particles. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula.
 Source: pinterest.com
Source: pinterest.com
From molesliter you can calculate mass in grams by converting the moles to grams using molar mass and then use the volume to calculate the final mass in that volume. From you calculate the molar mass to be 1021 gmol. The unit is typically gmol. Convert millilitres to grams. Formula to convert moles to grams.
 Source: youtube.com
Source: youtube.com
2 moles Ag 1 moles S 1 mole Ag 2S 2 1079 g 1321 g 1 2479 g 2479 g reactants 2479 g product. How to Convert Grams to Moles. A mole is defined as the number of substances containing particles of that substance as many as the atoms contained in 12000 grams of carbon atoms 12. 2 moles Ag 1 moles S 1 mole Ag 2S 2 1079 g 1321 g 1 2479 g 2479 g reactants 2479 g product. Ca NO_3_2 times frac 1.
 Source: youtube.com
Source: youtube.com
Calculate the volume of carbon dioxide gas CO 2 occupied by a 5 moles and b 05 moles of the gas occupied at STP. Find molar mass of krypton molar mass 838 gmol 2. The unit is typically gmol. After about a semester and a half I had forgotten how to convert from grams to molesThis article was extremely clear precise and definitive. Since moles grams of compoundthe molar mass to find the mass you will need to do mass molar mass x molarity.
 Source: pinterest.com
Source: pinterest.com
A gramg is a metric unit of mass and was formally defined as the absolute weight of a volume of pure water equal to the cube of the hundredth part of a meter and at the temperature of melting ice. Set up Equation Density gL molar mass molar volume Density 838𝑔 𝑜 224𝐿 𝑜 or Density 838 g1 mol x 1 mol 224 L Density of Kr 374 gL. 2 moles Ag 1 moles S 1 mole Ag 2S 2 1079 g 1321 g 1 2479 g 2479 g reactants 2479 g product. G dm3 Mass of solute in grams Vol. A mole is defined as the number of substances containing particles of that substance as many as the atoms contained in 12000 grams of carbon atoms 12.
 Source: youtube.com
Source: youtube.com
A Volume of CO 2 number of moles of CO 2 224 L 5 224. N m M where M is the molar mass of this material. Set up Equation Density gL molar mass molar volume Density 838𝑔 𝑜 224𝐿 𝑜 or Density 838 g1 mol x 1 mol 224 L Density of Kr 374 gL. Gas volumes from moles and grams. For example one molecule of H2O has two atoms of Hydrogen H and one atom of Oxygen O.
 Source: pinterest.com
Source: pinterest.com
From you calculate the molar mass to be 1021 gmol. While a mole is the unit of measurement for amount of substance in the International System of Units. From compound to compound to a number of atoms in a molecule vary. 100 mL 1082 g1 mL 108 g. Molar Concentration to Mass Concentration conversion Formula to calculate pH from molarity.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to find moles from grams and volume by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






