Your How to find mole ratio of a compound images are ready. How to find mole ratio of a compound are a topic that is being searched for and liked by netizens now. You can Find and Download the How to find mole ratio of a compound files here. Find and Download all free photos.
If you’re searching for how to find mole ratio of a compound images information linked to the how to find mole ratio of a compound topic, you have visit the right blog. Our website frequently provides you with suggestions for downloading the maximum quality video and picture content, please kindly search and locate more enlightening video content and graphics that match your interests.
How To Find Mole Ratio Of A Compound. In the second case you can usually find the mole ratio by balancing the equation for the reaction. Mass percent mass of element in 1 mole of compound mass of 1 mole of compound x 100. Mass percentage is calculated as the mass of a component divided by the total mass of the mixture multiplied by 100. Remember that percentages are a.
 Question Video Deducing The Molar Ratio Of Reactants From A Balanced Reaction Equation Nagwa From nagwa.com
Question Video Deducing The Molar Ratio Of Reactants From A Balanced Reaction Equation Nagwa From nagwa.com
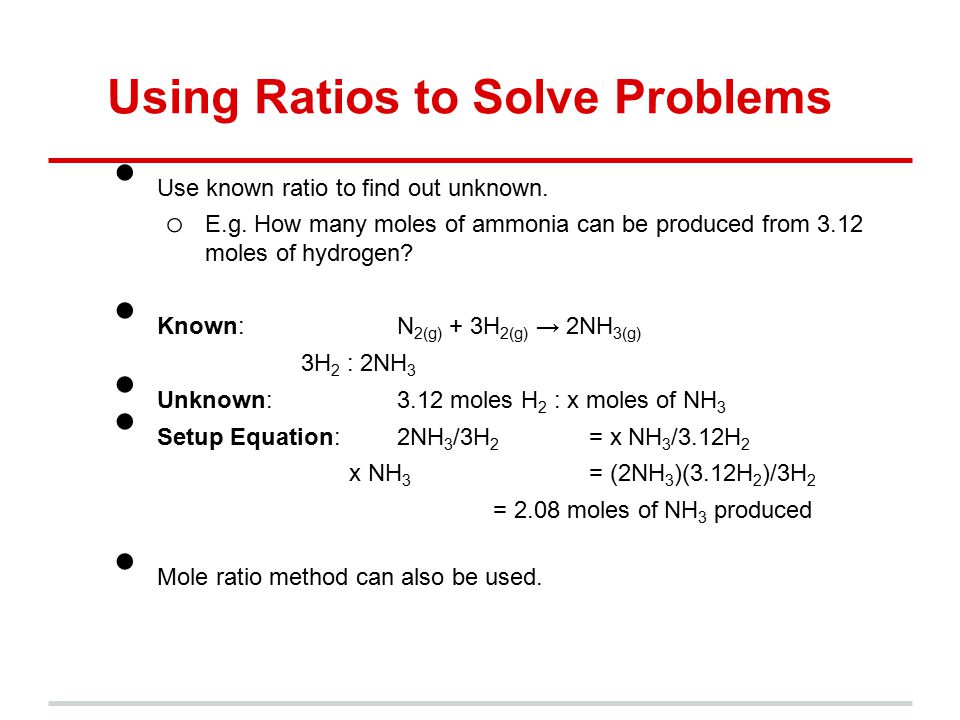
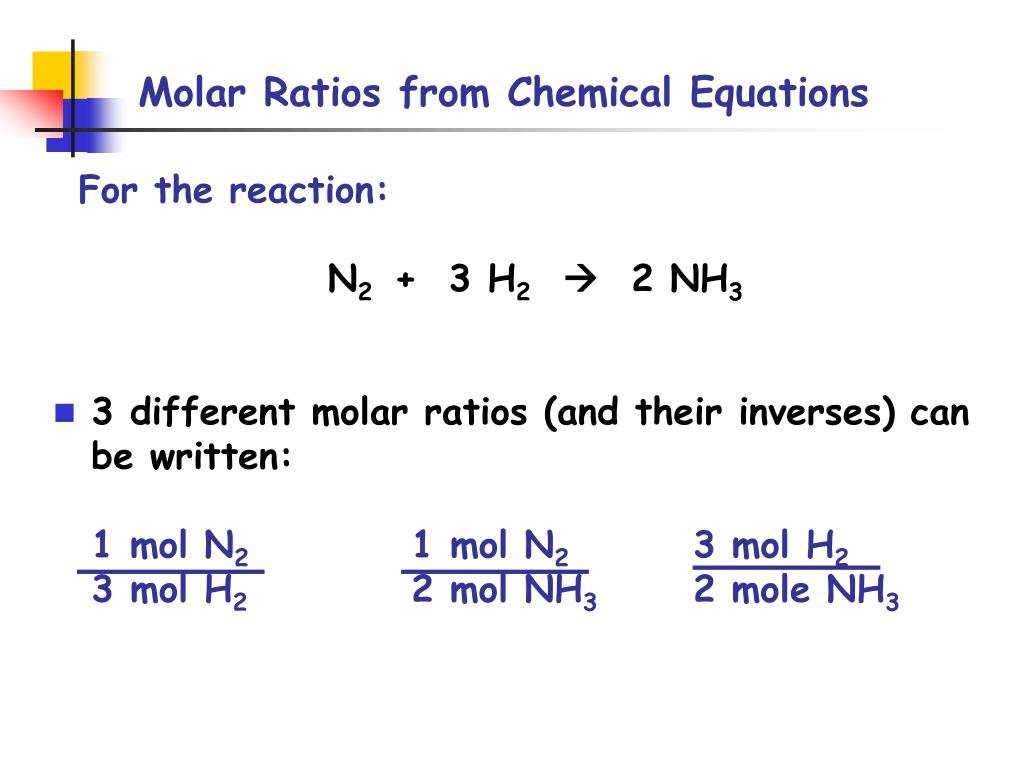
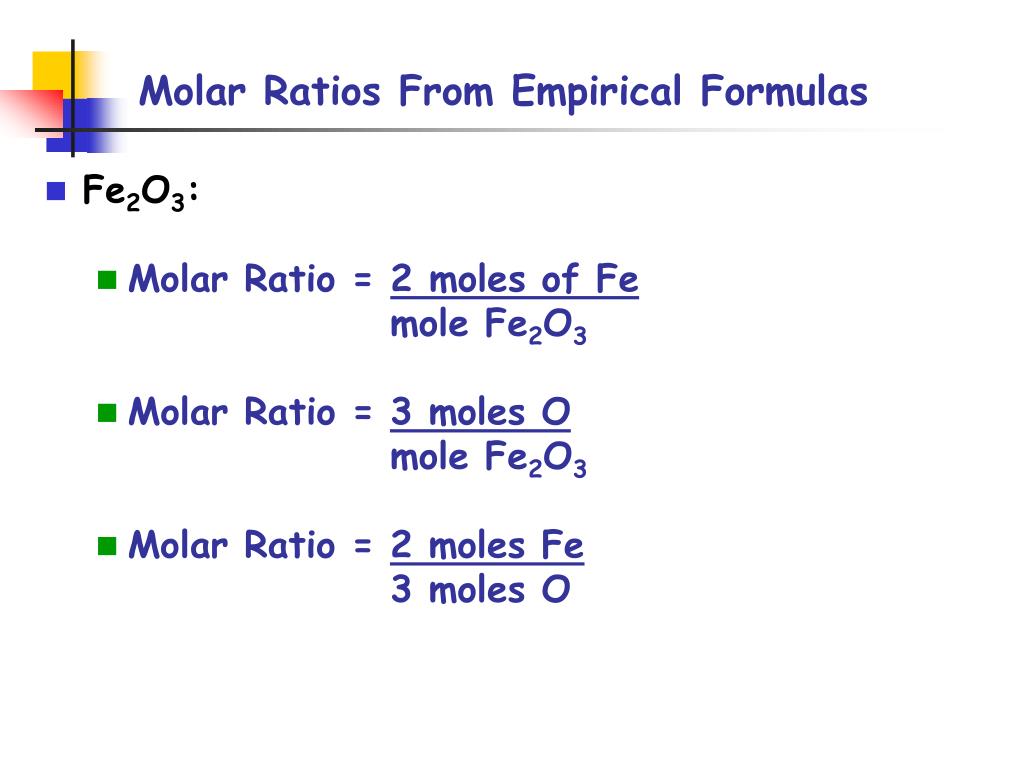
Lets say you have a mixture of components 1 and 2 that is 40 mole percent mole fraction 04 component one approximately the blue line labeled liquid on the diagram. One mole of a compound contains Avogadros number 6022 x 10 23 of molecules molecular compound or formula units ionic compoundThe molar mass of a compound tells you the mass of 1 mole of that substance. Moles O 545 g O x 1 mol O 1600 g O 341 mol O. The typical procedure to determine the empirical formula of a mystery compound is to analyze it for its component elements. CarbonHydrogen 14 Step 2. Mole ratios are important because mole ratios allow you change moles of a substance to moles of another substance.
Moles O 545 g O x 1 mol O 1600 g O 341 mol O.
From the coefficients of the equation the mole ratio is 33. Compare the amount of the each element in the compound. 2 mol O 2. Mass percent mass of element in 1 mole of compound mass of 1 mole of compound x 100. Divide moles of water by the moles of anhydrate to get the mole ratio. From the coefficients of the equation the mole ratio is 33.
 Source: youtube.com
Source: youtube.com
Mass percent mass of element in 1 mole of compound mass of 1 mole of compound x 100. This gives you a molar ratio of Al to I2 of 004448 0009456. Use every parts molar mass to transform the grams of every ingredient to moles. Divide the mass of anhydrate by the molar mass of anhydrate to get moles of anhydrate. 1 mol CO 2.
 Source: msjchem.com
Source: msjchem.com
In the second case you can usually find the mole ratio by balancing the equation for the reaction. 1 mol CO 2. However this reduces to a 11 ratio. Find the number of atoms of. Then find the ratio of the masses of copper in the two compounds by dividing the larger value.
 Source: quizlet.com
Source: quizlet.com
2 mol O 2. A 25 to 1 is really a 5 to 2 ratio. Divide moles of water by the moles of anhydrate to get the mole ratio. In other words 1 mol of methane will produced 1 mole of carbon dioxide as long as the reaction goes to completion and there is plenty of oxygen present. 1 mol CO 2.
 Source: slideplayer.com
Source: slideplayer.com
This gives you a molar ratio of Al to I2 of 004448 0009456. The typical procedure to determine the empirical formula of a mystery compound is to analyze it for its component elements. Round off the mole ratio to the nearest whole number if less than 019 or greater than 081 fractions. Mass percentage is calculated as the mass of a component divided by the total mass of the mixture multiplied by 100. Otherwise multiply the mole ratio by a number which gives two whole numbers for the ratio within 01 range of the whole number.
 Source: slideplayer.com
Source: slideplayer.com
Mass percent grams of solute grams of solute plus solvent x 100. Well look at several simple ways to find the mole. Also determine the water in the hydrate. In other words 1 mol of methane will produced 1 mole of carbon dioxide as long as the reaction goes to completion and there is plenty of oxygen present. In other words it tells you the number of.
 Source: youtube.com
Source: youtube.com
F is the number of reactive groups per mole of compound. Also determine the water in the hydrate. Lets say you have a mixture of components 1 and 2 that is 40 mole percent mole fraction 04 component one approximately the blue line labeled liquid on the diagram. For elements eq6times 1023 eq atoms equals one mole and for compounds one mole is eq6times1023 eq molecules of the compound. These molar ratios can also be expressed as fractions.
 Source: youtube.com
Source: youtube.com
These molar ratios can also be expressed as fractions. Round off the mole ratio to the nearest whole number if less than 019 or greater than 081 fractions. To find the simplest whole number ratio divide each number by the smallest number of moles. The mole ratio is the magic that changes from A to B. Use every parts molar mass to transform the grams of every ingredient to moles.
 Source: slideplayer.com
Source: slideplayer.com
Get the mass of each element by assuming a certain overall mass for thesample 100 g is a good mass to assume when working with percentages. Mass percent grams of solute grams of solute plus solvent x 100. 100 g n 2 x 1 mole n 2 280 g n 2 0357 moles n 2 have 100 g h 2 x 1 mole h 2 202 g h 2 495 moles h 2 have step 3. Here are a number of highest rated How To Calculate Mole Ratio pictures upon internet. 2 mol H 2 O.
 Source: slideserve.com
Source: slideserve.com
F is the number of reactive groups per mole of compound. Mass percent grams of solute grams of solute plus solvent x 100. For each compound find the grams of copper that combine with 100 g of chlorine by dividing the mass of copper by the mass of chlorine. Find the number of atoms of. Please note that using a 33 ratio in a calculation is equivalent to using a 11 ratio.
 Source: youtube.com
Source: youtube.com
F is the number of reactive groups per mole of compound. For each one mole of CuSO 4 there are 5 moles of water. 2 mol O 2. Divide the mass of anhydrate by the molar mass of anhydrate to get moles of anhydrate. 151 g Na2 CO3 106 gmol Na2 CO3 0142 moles Na2 CO3.
 Source: nagwa.com
Source: nagwa.com
The ratio for the amount of oxygen in two moles of nitrogen dioxide is 2-to-2. In other words it tells you the number of. These molar ratios can also be expressed as fractions. Remember that percentages are a. How To Determine Limiting Reactant From Mole Ratio.
 Source: dummies.com
Source: dummies.com
From the coefficients of the equation the mole ratio is 33. F is the number of reactive groups per mole of compound. Compare the amount of the each element in the compound. The typical procedure to determine the empirical formula of a mystery compound is to analyze it for its component elements. Here are a number of highest rated How To Calculate Mole Ratio pictures upon internet.
 Source: slideserve.com
Source: slideserve.com
Also determine the water in the hydrate. CarbonHydrogen 14 Step 2. Theratio of the moles of each element will provide the ratio of the atoms ofeach element. That means that answer choice a would be considered by most teachers to be the correct answer. Divide moles of water by the moles of anhydrate to get the mole ratio.
 Source: nagwa.com
Source: nagwa.com
Round off the mole ratio to the nearest whole number if less than 019 or greater than 081 fractions. That means that answer choice a would be considered by most teachers to be the correct answer. Please note that using a 33 ratio in a calculation is equivalent to using a 11 ratio. Feb 09 2021 Steps to find out empirical system. From the coefficients of the equation the mole ratio is 33.
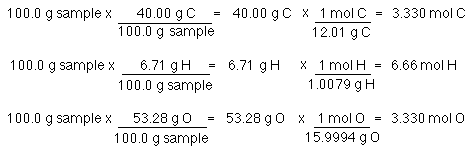 Source: westfield.ma.edu
Source: westfield.ma.edu
Usually you divide each number in the fraction by the smaller number of moles. Remember that percentages are a. For each one mole of CuSO 4 there are 5 moles of water. For every compound discover the grams of copper that mix with 100 g of chlorine by dividing the mass of copper by the mass of chlorine. Mass percent mass of element in 1 mole of compound mass of 1 mole of compound x 100.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to find mole ratio of a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






