Your How to find molar mass using grams images are available in this site. How to find molar mass using grams are a topic that is being searched for and liked by netizens now. You can Find and Download the How to find molar mass using grams files here. Find and Download all free photos and vectors.
If you’re looking for how to find molar mass using grams images information related to the how to find molar mass using grams topic, you have pay a visit to the ideal site. Our site always gives you suggestions for viewing the highest quality video and image content, please kindly hunt and locate more enlightening video articles and graphics that match your interests.
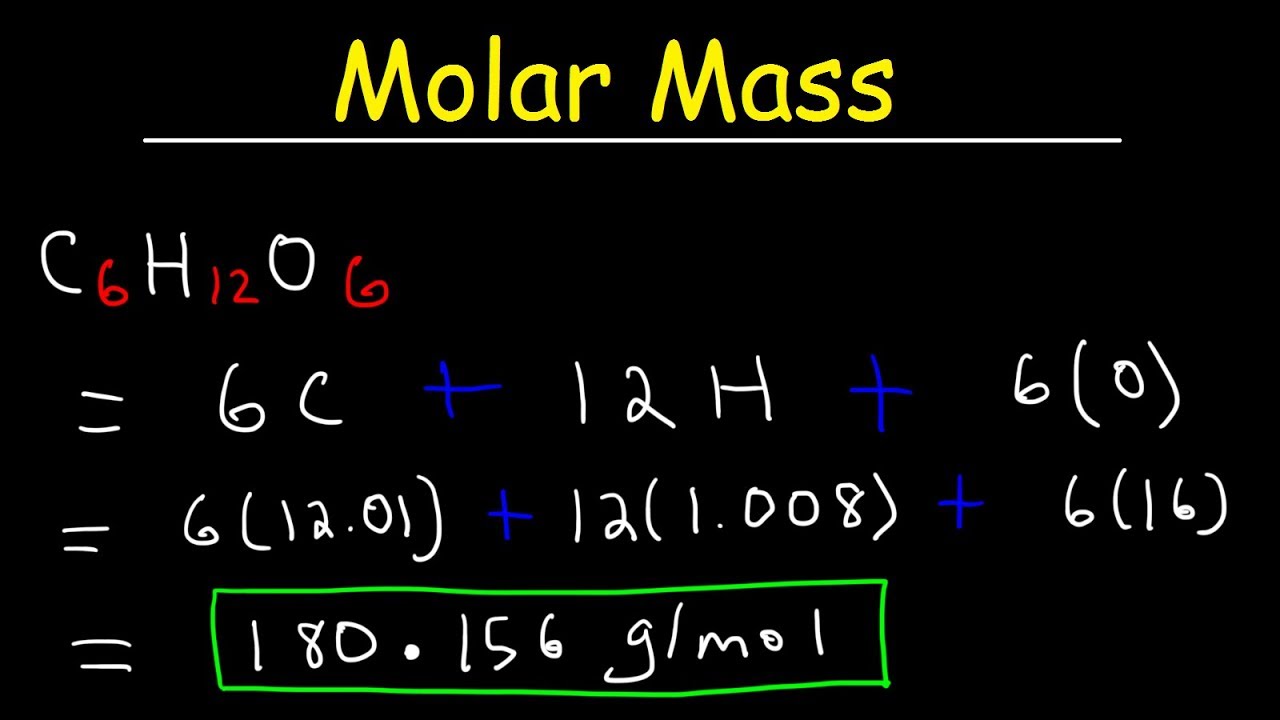
How To Find Molar Mass Using Grams. But if we want to express it in KgMole then devide it by 1000. The addition of a nonvolatile solute to a solvent causes the boiling point of the solvent to increase and the freezing point of the solvent to decrease. Find the molar mass of hydrogen from the periodic table. From the value of the molar mass you can tell that this gas is oxygen with the chemical formula O 2.
 Molar Mass Calculator Inch Calculator From inchcalculator.com
Molar Mass Calculator Inch Calculator From inchcalculator.com
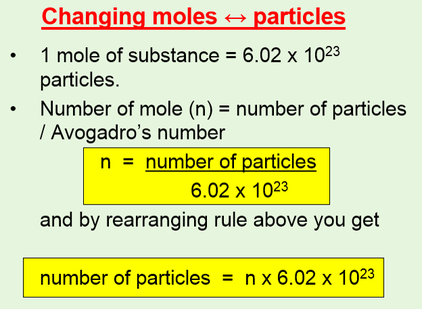
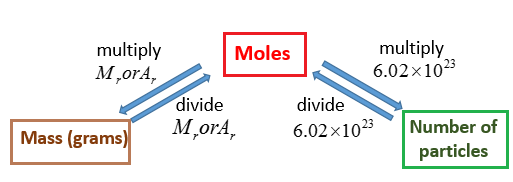
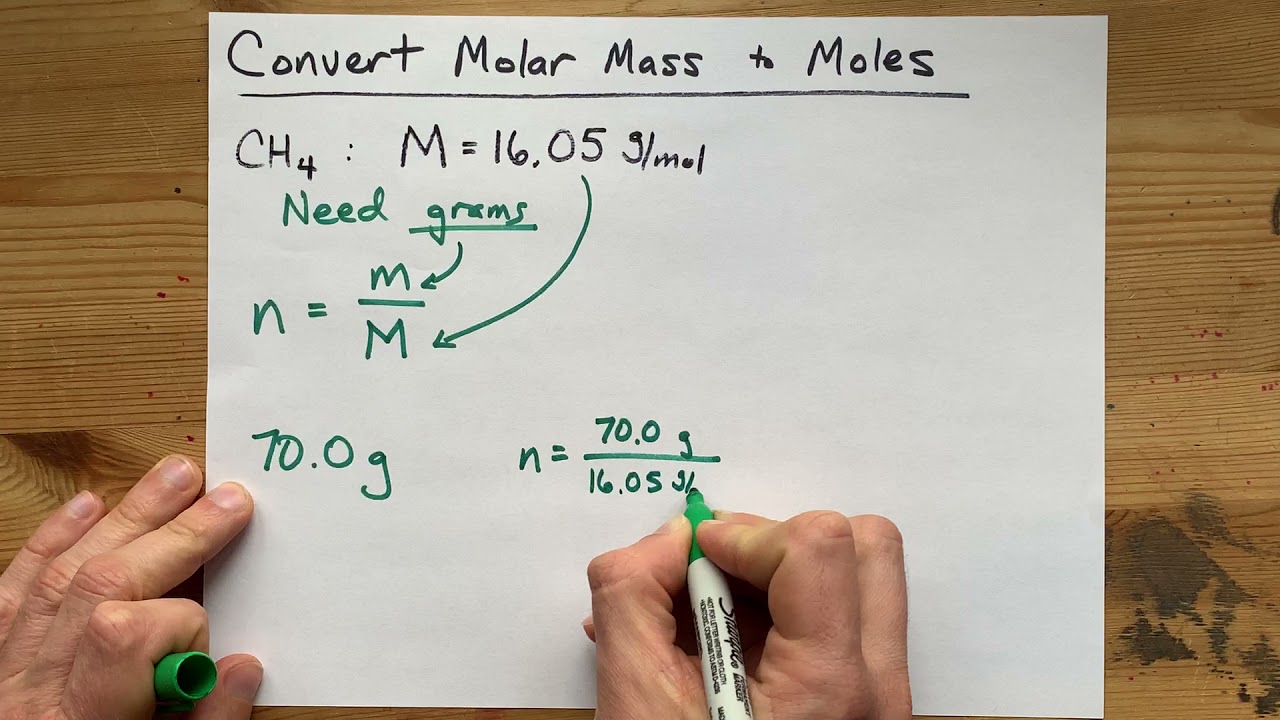
Moles relate the number of atoms in a sample to its mass in grams using molar mass which tells us the mass of one mole of atoms in a sample. More free chemistry help videos. Now we can use the rearranged equation. This converts atomic units to grams per mole. The unit is typically gmol. You can use Molar mass of the substance alone to calculate molar mass.
We can find the molar mass of a gas using the ideal.
Or multiply it by 10 -3 ten to the power -3. The formula for moles to grams is given by. Now use the number of moles and multiply it by the molar mass. Excerpted from The Complete Idiots Guide to Chemistry 2003 by Ian Guch. Calculate Grams from Molar Mass. 1348 g V 2580 ml 2580 x 10-3 dm 3 P 760 mm 760760 1 atm T 0 273 273 K.
 Source: inchcalculator.com
Source: inchcalculator.com
How do you convert moles to liters per molarity. Therefore the units of molar mass are gramsmoleHow to find the molar mass of a compound. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. To calculate the number of moles in a solution given the molarity we multiply the molarity by total volume of the solution in liters. We can therefore write that n m M which can be used in the ideal gas law equation to get the value of the gas molar mass.
 Source: clutchprep.com
Source: clutchprep.com
Now use the number of moles and multiply it by the molar mass. Determine the moles of unknown the solute from the molarityof the solution and the volume in liters of the solution. Moles mol x Molar Mass gmol 1 x 5844. And it is 32 103 Kg Mole. We will look at compounds containing polyatomic ions and also hydrate compounds.
 Source: youtube.com
Source: youtube.com
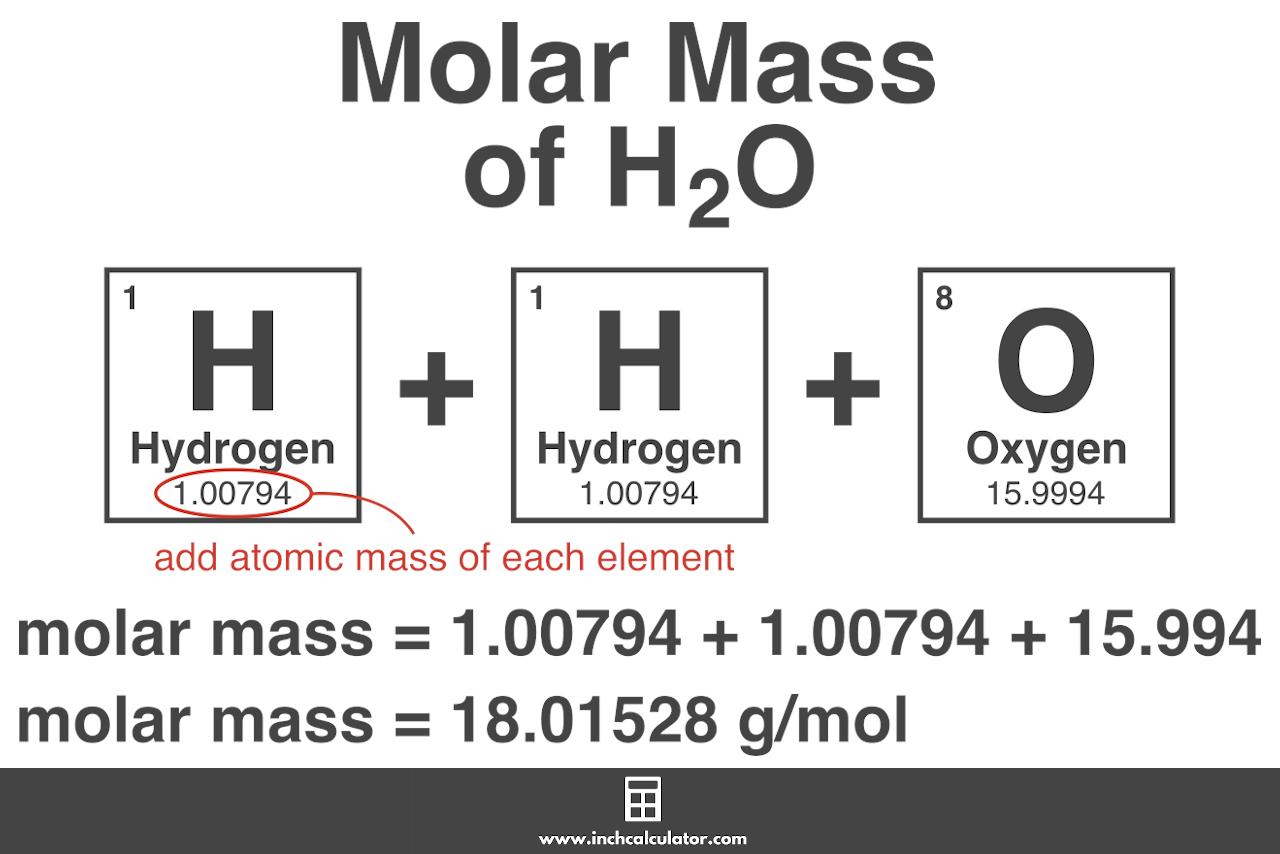
Excerpted from The Complete Idiots Guide to Chemistry 2003 by Ian Guch. Add up all and assign unit as gramsmole. Determine the molar mass from the mass of the unknown and thenumber of moles of unknown. From the value of the molar mass you can tell that this gas is oxygen with the chemical formula O 2. This is defined as 0001 kilogram per mole or 1 gram per mole.
 Source: youtube.com
Source: youtube.com
Look at the periodic table to determine the atomic mass of each of the. M - the gas mass in grams. More free chemistry help videos. The unit is typically gmol. The formula of this compound is C Molar mass can be used as a conversion factor for converting grams to moles and moles to grams.
 Source: wisc.pb.unizin.org
Source: wisc.pb.unizin.org
First you will need to calculate the molar mass of calcium bromide by using the periodic table and the number of each element in the formula. How many grams are in 379 moles of calcium bromide CaBr 2. You can use Molar mass of the substance alone to calculate molar mass. Look at the periodic table to determine the atomic mass of each of the. To calculate the number of moles in a solution given the molarity we multiply the molarity by total volume of the solution in liters.
 Source: onlinemathlearning.com
Source: onlinemathlearning.com
The addition of a nonvolatile solute to a solvent causes the boiling point of the solvent to increase and the freezing point of the solvent to decrease. We will look at compounds containing polyatomic ions and also hydrate compounds. Mass g No. Causey shows you step by step how to calculate the grams of copper II chloride from moles copper II chloride using t. The formula for moles to grams is given by.

By ideal gas equation PV nRT. Molar mass of Oxygen is 32 gram mole. For NaCl the molar mass is 5844 gmol. The unit is typically gmol. N - the number of moles of gas.
 Source: youtube.com
Source: youtube.com
How many grams are in 379 moles of calcium bromide CaBr 2. We can find the molar mass of a gas using the ideal. You can use Molar mass of the substance alone to calculate molar mass. Molar mass is generally expressed in gramsmole. The formula of this compound is C Molar mass can be used as a conversion factor for converting grams to moles and moles to grams.
 Source: youtube.com
Source: youtube.com
Find the molar mass of hydrogen from the periodic table. Determine the molar concentration of the unknown in the solutionfrom the observed osmotic pressure. Find out the molar mass of the substance hint. Finding molar mass also called molecular weight molecular mass and gram formula mass is an essential skill in chemistry especially for mole to gram conv. Add up all and assign unit as gramsmole.
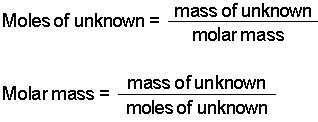 Source: chem.purdue.edu
Source: chem.purdue.edu
From the value of the molar mass you can tell that this gas is oxygen with the chemical formula O 2. Molar mass is a quantity that is very similar to molecular mass molecular weight formula mass and formula weight. Molar mass grams moles so we need to find the grams and divide that by the number of moles. 1348 g V 2580 ml 2580 x 10-3 dm 3 P 760 mm 760760 1 atm T 0 273 273 K. Moles mol x Molar Mass gmol 1 x 5844.
 Source: slideplayer.com
Source: slideplayer.com
Look at the formula to determine how many atoms of each type are in it. Calculating molar mass. In other words the molar mass is the total mass of all the atoms in grams that make a mole of a particular molecule. M m n where. Now we can use the rearranged equation.
 Source: youtube.com
Source: youtube.com
Moles mol x Molar Mass gmol 1 x 5844. M m RT P V. Determine the molar mass from the mass of the unknown and thenumber of moles of unknown. To calculate molar mass. Moles mol x Molar Mass gmol 1 x 5844.
 Source: khanacademy.org
Source: khanacademy.org
N - the number of moles of gas. Determine the molar concentration of the unknown in the solutionfrom the observed osmotic pressure. And it is 32 103 Kg Mole. Example 1 Calculate the mass in grams of 36 mol of H2SO4. Make use of the chemical formula to determine the number of atoms of each element in the compound.
 Source: youtube.com
Source: youtube.com
Multiply the molar mass of hydrogen by 2 the subscript that follows the H. M m n where. First you will need to calculate the molar mass of calcium bromide by using the periodic table and the number of each element in the formula. Find out the molar mass of the substance hint. N m M where M is the molar mass of this material.
 Source: slideserve.com
Source: slideserve.com
But if we want to express it in KgMole then devide it by 1000. Molar mass of Oxygen is 32 gram mole. First you will need to calculate the molar mass of calcium bromide by using the periodic table and the number of each element in the formula. N m M where M is the molar mass of this material. We can find the molar mass of a gas using the ideal.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find molar mass using grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.