Your How to find molar mass of a compound example images are available in this site. How to find molar mass of a compound example are a topic that is being searched for and liked by netizens now. You can Download the How to find molar mass of a compound example files here. Download all free photos.
If you’re looking for how to find molar mass of a compound example pictures information related to the how to find molar mass of a compound example topic, you have visit the ideal site. Our site always gives you suggestions for viewing the highest quality video and picture content, please kindly surf and locate more informative video articles and images that fit your interests.
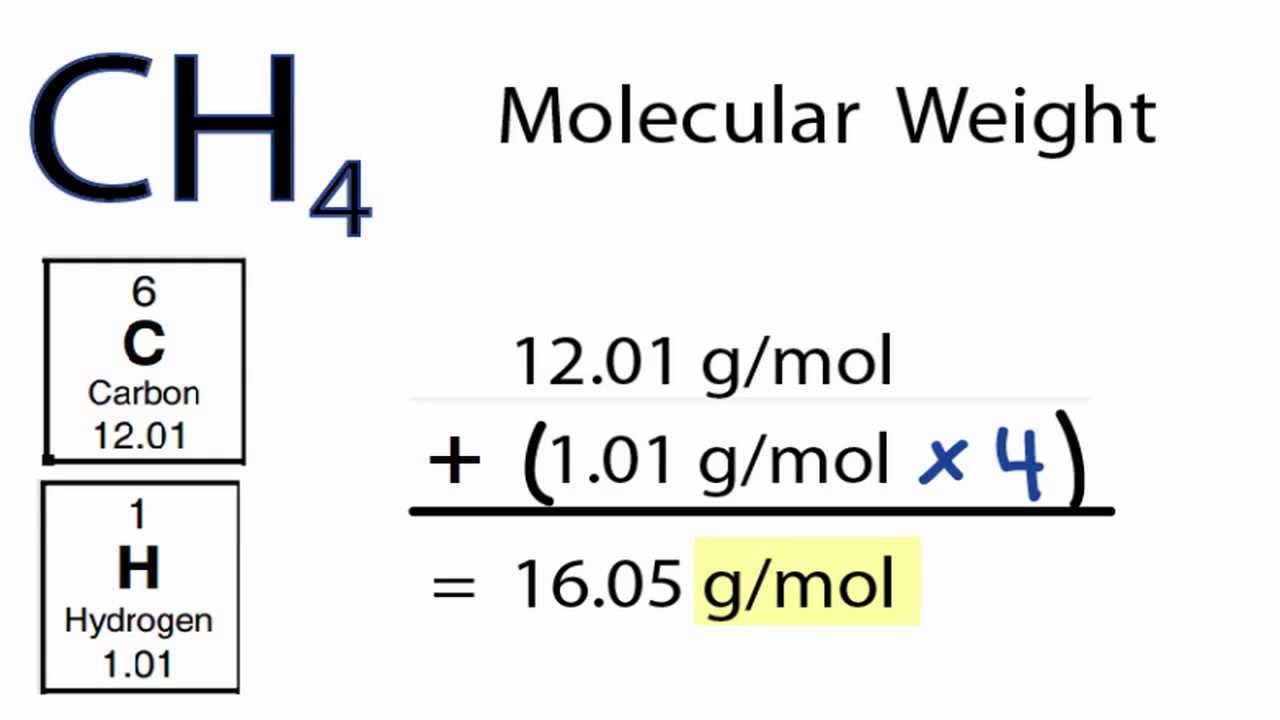
How To Find Molar Mass Of A Compound Example. Connect the atomic masses atomic weights of all atoms within the molecule to calculate the molar mass. So its going to be six times 1201 grams per mole plus 12 times 1008 grams per mole plus every molecule of glucose has six oxygen plus six times 1600 grams per mole. 2 x 1008 g hydrogen 1 x 1600 g oxygen 1802 g. Add up all and assign unit as gramsmole.
 Molar Mass Molecular Weight Of Ch4 Methane Youtube From youtube.com
Molar Mass Molecular Weight Of Ch4 Methane Youtube From youtube.com
The molar mass of sodium is 2299 gmol oxygen is. 316 gmol 48 gmol. In 4788 grams of titanium there is one mole or 6022 x 10 23 titanium atoms. Add together the atomic masses of all of the atoms of hydrogen and water in a molecule of water. So its going to be six times 1201 grams per mole plus 12 times 1008 grams per mole plus every molecule of glucose has six oxygen plus six times 1600 grams per mole. For example the molar mass of NaCl can be calculated for finding the atomic mass of sodium 2299 gmol and the atomic mass of chlorine 3545 gmol and combining them.
And therefore the mass of chlorine 3545 gmol and mixing them.
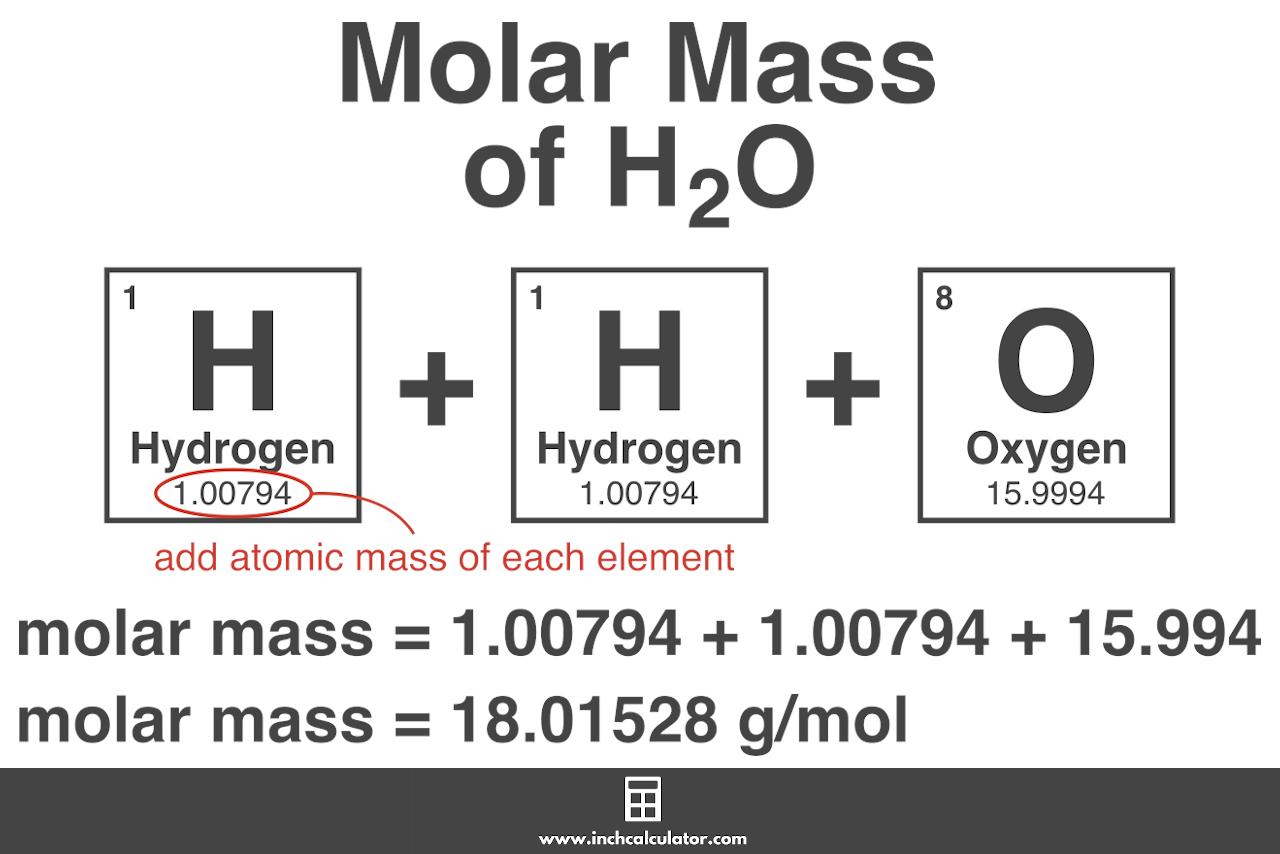
Therefore the units of molar mass are gramsmoleHow to find the molar mass of a compound. For hydrogen chloride the molar mass is 1007 35453 36460 gmol. To find the molar mass of a chemical you have to bring the molar mass of all of the components in that chemical. Therefore the molar mass of H 2 O is 18 gmol. Finding the molar mass of a compound is one of the most basic abilities a chemist. Apply the same principles to calculate the molar mass of a molecule.
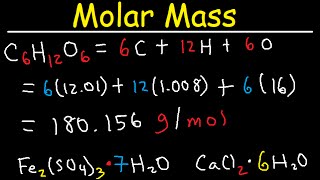 Source: youtube.com
Source: youtube.com
First black basketball player in nba. For example the molar mass of NaCl can be calculated for finding the atomic mass of sodium 2299 gmol and the atomic mass of chlorine 3545 gmol and combining them. The molar mass of sodium is 2299 gmol oxygen is. How do you calculate the molar mass of an acid. Molecular mass 1 x 140067 3 x 100794 molecular mass 140067 302382.
 Source: khanacademy.org
Source: khanacademy.org
So the molar mass of glucose is going to be six times the molar mass of carbon plus 12 times the molar mass of hydrogen plus six times the molar mass of oxygen. Next multiply the atomic mass of each atom by the number of atoms in the compound. One mole of H 2 O is composed of 6023 x 10 23 H 2 O molecules. HOWEVER make sure that you use at least as many significant. Capitalize the first letter in chemical symbol and use lower case for the remaining letters.
 Source: tutorpace.com
Source: tutorpace.com
What is a tefl qualification equivalent to. Find the molar mass of sodium carbonate Na 2CO 3. Its easy to find the molecular mass of a compound with these steps. As a result we would say that the molar mass of sulfuric acid is 9809 gmol. Na 2 x 230 460 C 1 x 120 120 O 3 x 160 480 molar mass 1060 gmole For many but not all problems you can simply round the atomic weights and the molar mass to the nearest 01 gmole.
 Source: youtube.com
Source: youtube.com
Molar mass is the mass of a given substance divided by the amount of that substance measured in gmol. 1Ca 1S 4008 gmol 3206 gmol 7214 gmol. For hydrogen chloride the molar mass is 1007 35453 36460 gmol. Its easy to find the molecular mass of a compound with these steps. Molar mass is the mass of a given substance divided by the amount of that substance measured in gmol.
 Source: youtube.com
Source: youtube.com
To calculate the molar mass of a compound with multiple atoms sum all the mass of the constituent atoms. One mole of H 2 O is composed of 6023 x 10 23 H 2 O molecules. So its going to be six times 1201 grams per mole plus 12 times 1008 grams per mole plus every molecule of glucose has six oxygen plus six times 1600 grams per mole. Have a look at NaOH which contains sodium oxygen and hydrogen. 2 x 1008 g hydrogen 1 x 1600 g oxygen 1802 g.
 Source: inchcalculator.com
Source: inchcalculator.com
What is a tefl qualification equivalent to. All you need to do is find the atomic mass of the element on the periodic table and report the number with the unit grams per mole or gmol. To find the molar mass of a chemical you have to bring the molar mass of all of the components in that chemical. One mole of H 2 O is composed of 6023 x 10 23 H 2 O molecules. This chemistry video tutorial explains how to calculate the molar mass of a compound.
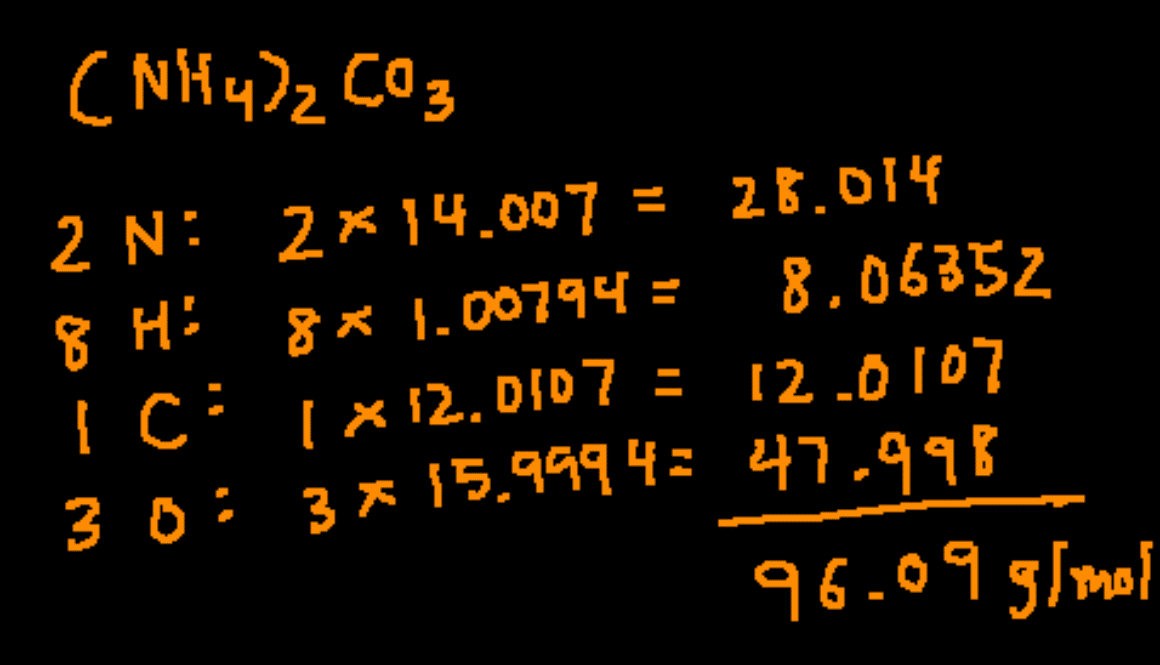 Source: conquerchemistry.com
Source: conquerchemistry.com
The molar mass of NaCl is 5844 gmol. Molar mass is the mass of a given substance divided by the amount of that substance measured in gmol. Therefore the molar mass of H 2 O is 18 gmol. The molar mass of NaCl is 5844 gmol. Multiply each elements atomic mass by the number of atoms of that element in the molecule.
 Source: onlinemathlearning.com
Source: onlinemathlearning.com
To calculate the molar mass of a compound with multiple atoms sum all the mass of the constituent atoms. For glucose the molar mass is 720642. For example the calculation of the molar mass of NaCl is for locating the mass of sodium 2299 gmol. Multiply the atomic weight of each element with its number of atoms present in the compound. The molar mass of calcium sulfide is 7214 gmol.
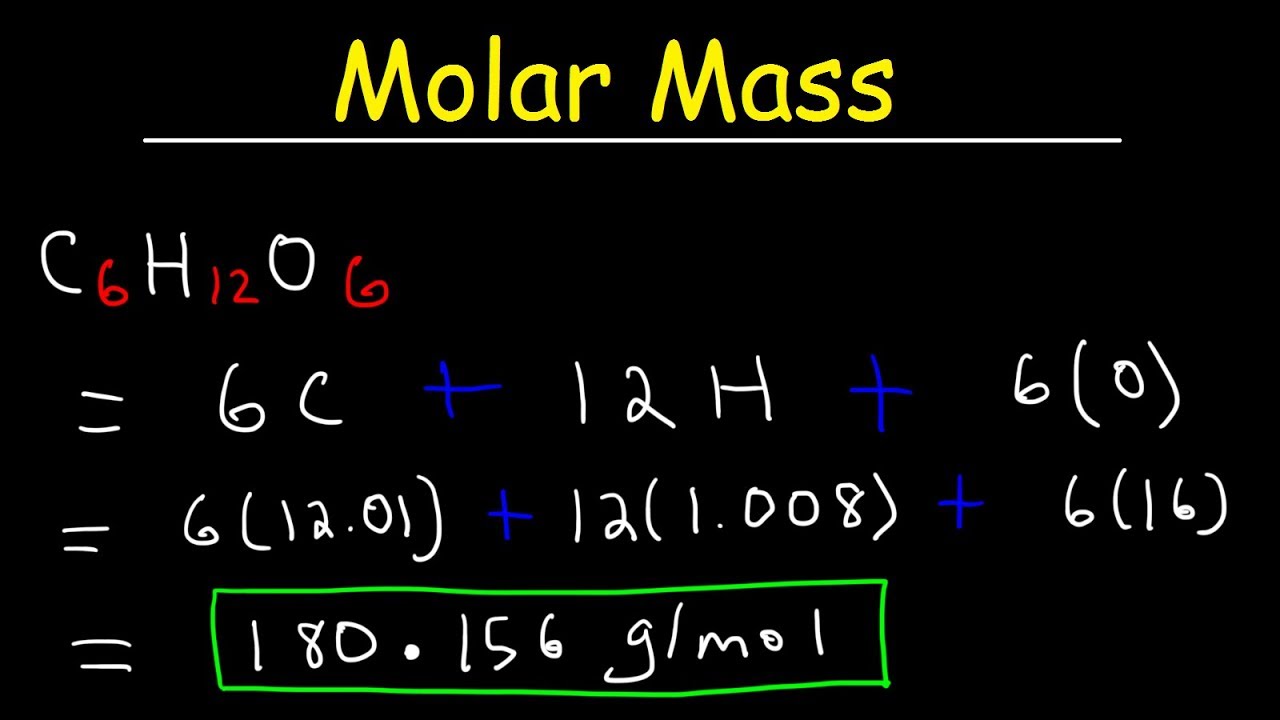 Source: youtube.com
Source: youtube.com
Therefore the units of molar mass are gramsmoleHow to find the molar mass of a compound. Find the atomic mass for each element using the mass shown in the Periodic Table or Atomic Weight Table. Molar mass is the mass of a given substance divided by the amount of that substance measured in gmol. What is a tefl qualification equivalent to. To calculate molar mass of a chemical compound please enter its chemical formula and click Calculate.
 Source: pediaa.com
Source: pediaa.com
Multiply the atomic weight of each element with its number of atoms present in the compound. 3646 grams is the mass of one mole of hydrogen chloride. In chemical formula you may use. This chemistry video tutorial explains how to calculate the molar mass of a compound. Have a look at NaOH which contains sodium oxygen and hydrogen.
 Source: clutchprep.com
Source: clutchprep.com
Find the atomic mass for each element using the mass shown in the Periodic Table or Atomic Weight Table. As a result we would say that the molar mass of sulfuric acid is 9809 gmol. It contains plenty of examples and practice problemsMy E-Book. For example the calculation of the molar mass of NaCl is for locating the mass of sodium 2299 gmol. 2 x 1008 g hydrogen 1 x 1600 g oxygen 1802 g.
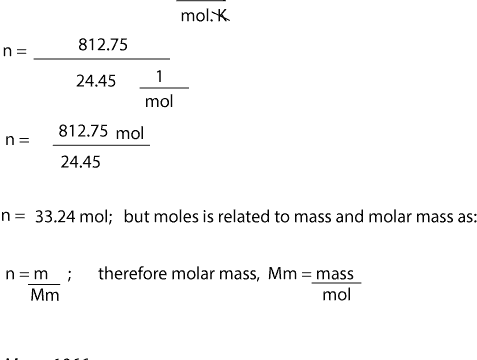 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
Apply the same principles to calculate the molar mass of a molecule. When you add these numbers up you come up with the molar mass of the compound. Finding the molar mass of a compound is one of the most basic abilities a chemist. Find the atomic mass for each element using the mass shown in the Periodic Table or Atomic Weight Table. Ca Fe Mg Mn S O H C N Na K Cl Al.

This chemistry video tutorial explains how to calculate the molar mass of a compound. There is one nitrogen atom no subscript is given for one atom. The molar mass of calcium sulfide is 7214 gmol. As a result we would say that the molar mass of sulfuric acid is 9809 gmol. This chemistry video tutorial explains how to calculate the molar mass of a compound.
 Source: flexbooks.ck12.org
Source: flexbooks.ck12.org
1Ca 1S 4008 gmol 3206 gmol 7214 gmol. It contains plenty of examples and practice problemsMy E-Book. From this you can see that sodiums molar mass will be 2299 gmol. Definitions of molecular mass molecular weight molar mass and molar weight. 2 x 1008 g hydrogen 1 x 1600 g oxygen 1802 g.
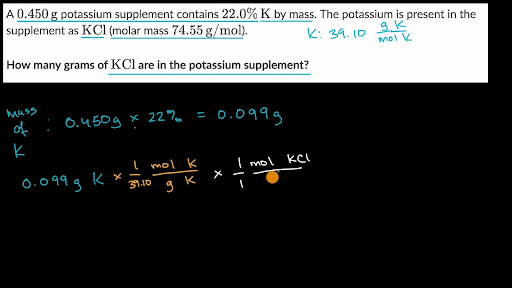
Multiply each elements atomic mass by the number of atoms of that element in the molecule. HOWEVER make sure that you use at least as many significant. It contains plenty of examples and practice problemsMy E-Book. Molecular mass 170305. Its easy to find the molecular mass of a compound with these steps.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to find molar mass of a compound example by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






