Your How to find molar mass in grams per mole images are available in this site. How to find molar mass in grams per mole are a topic that is being searched for and liked by netizens now. You can Get the How to find molar mass in grams per mole files here. Download all royalty-free images.
If you’re searching for how to find molar mass in grams per mole pictures information linked to the how to find molar mass in grams per mole keyword, you have visit the ideal blog. Our website frequently provides you with suggestions for seeing the maximum quality video and image content, please kindly search and locate more enlightening video content and images that fit your interests.
How To Find Molar Mass In Grams Per Mole. Convert grams Salicylic Acid to moles or moles Salicylic Acid to grams. How many grams are in 379 moles of calcium bromide CaBr 2. Do coefficients affect molar mass. Convert moles Co2 to grams - Conversion Step 3.
 Determine The Mass Of Each Of The Followin Clutch Prep From clutchprep.com
Determine The Mass Of Each Of The Followin Clutch Prep From clutchprep.com
It is the amount of grams that is numerically equal to the molecular weight. The molar mass of a substance is the mass in grams of 1 mole of the substance. First you must calculate the number of moles in this solution by rearranging the equation. Since the unified atomic mass unit symbol. When finding the molar mass of the reactants you use the number of moles given in the problem and multiply it by the molar mass of that element or compound to get its mass. Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom.
First divide by 1000 to convert to gramsin this case 01 grams.
Mass g No. Now we can use the rearranged equation. Add up all and assign unit as gramsmole. When finding the molar mass of the reactants you use the number of moles given in the problem and multiply it by the molar mass of that element or compound to get its mass. U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da. The formula for moles to grams is given by.
 Source: slideplayer.com
Source: slideplayer.com
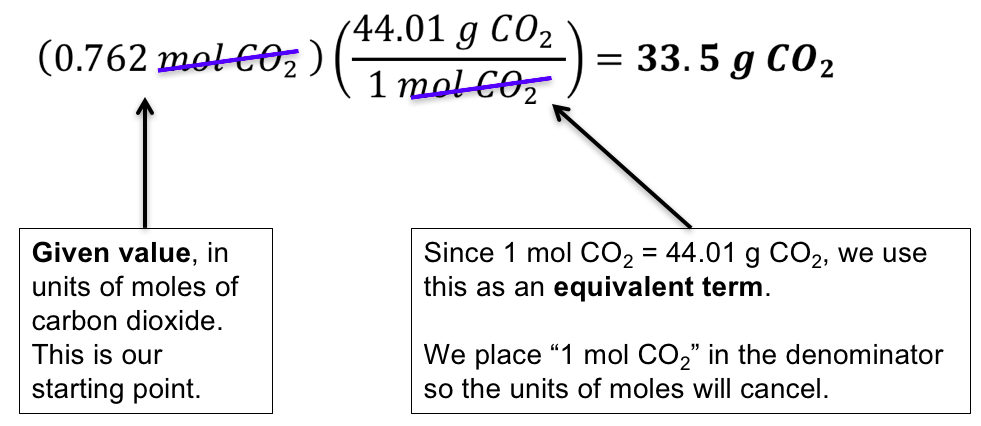
Molecular Weight To Moles - 9 images - molar mass molecular weight of n2o youtube how to convert grams to moles video lesson transcript. Moles to Grams Conversion Formula Questions. It is the amount of grams that is numerically equal to the molecular weight. Therefore Carbon Dioxide has a molar mass of 4399 grams per mole. Add up all and assign unit as gramsmole.
 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
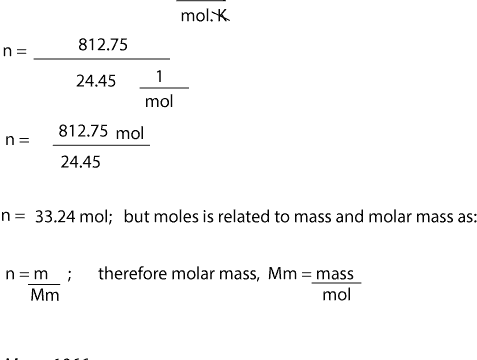
1 mole is equal to 1 moles Glycerol or 9209382 grams. Example 1 Calculate the mass in grams of 36 mol of H2SO4. For example if you want to find the molar mass of carbon you would find the atomic mass of carbon on the periodic table and this is equal to the molar mass in grams per mole. NaCl 229898 gL 354530 gL. Therefore the units of molar mass are gramsmoleHow to find the molar mass of a compound.
 Source: khanacademy.org
Source: khanacademy.org
Convert moles Co2 to grams - Conversion Step 3. Calculating mass ratios is easy enough if you. NaCl 229898 gL 354530 gL. The mole and molar mass. For a molecule with a weight of 32 the molar mass is 32 grams.
 Source: slideplayer.com
Source: slideplayer.com
For a molecule with a weight of 32 the molar mass is 32 grams. Always multiply the subscript by the atomic mass and when its done where it equals 3198 grams. The molar mass is the mass of one mole of a substance. Molecular Weight To Moles - 9 images - molar mass molecular weight of n2o youtube how to convert grams to moles video lesson transcript. 2 1008 3206 4 18 106076.
 Source: youtube.com
Source: youtube.com
Lets figure out what the difference between molar mass and atomic mass is and learn to use molar mass as a conversion factor and stop guessing on how to con. Calculating mass ratios is easy enough if you. Therefore the mole is a unit for that physical quantity. So the molar mass for AgNO₃ is ____ grams. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl.
 Source: youtube.com
Source: youtube.com
U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da. Oxygen has a subscript of 2 in this element and has an atomic mass of 1599 grams. Calculating mass ratios is easy enough if you. Experiments counting the number of 12 C atoms in a 12-gram sample have determined. First you must calculate the number of moles in this solution by rearranging the equation.

The molar mass is the mass of one mole of a substance. We can then use the calculated molar mass to convert between mass and number of moles of the substance. Make use of the chemical formula to determine the number of atoms of each element in the compound. The mole and molar mass. Then divide this amount by the molarmass which represents the number of grams in a mole.
 Source: clutchprep.com
Source: clutchprep.com
NaCl 229898 gL 354530 gL. Its unit is in grams per mole. Moles mol Molarity M x Volume L 05 x 2. So in our example carbon has a molar mass of 1201 grams per mole. First you must calculate the number of moles in this solution by rearranging the equation.
 Source: clutchprep.com
Source: clutchprep.com
Therefore the molecular mass of H2SO4 is. Example 1 Calculate the mass in grams of 36 mol of H2SO4. The mole and molar mass. Moles mol Molarity M x Volume L 05 x 2. Multiply the atomic weight of each element with its number of atoms present in the compound.
 Source: wisc.pb.unizin.org
Source: wisc.pb.unizin.org
When finding the molar mass of the reactants you use the number of moles given in the problem and multiply it by the molar mass of that element or compound to get its mass. Calculating mass ratios is easy enough if you. So the molar mass for AgNO₃ is ____ grams. Therefore the mole is a unit for that physical quantity. Now use the number of moles and multiply it by the molar mass.
 Source: youtube.com
Source: youtube.com
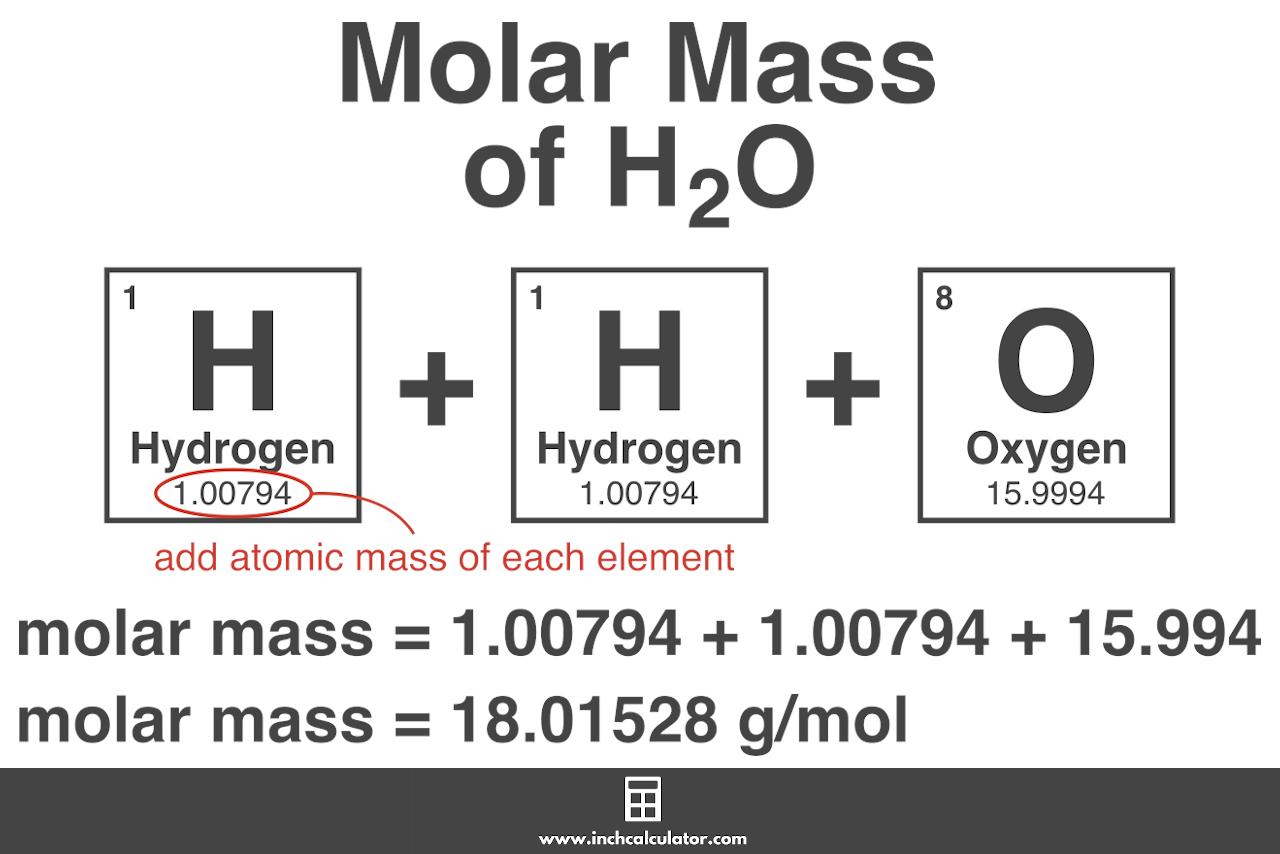
The units of molar mass are grams per mole abbreviated as gmol. The unit is typically gmol. For O2 thisis approximately 319988. NaCl Na Cl. Experiments counting the number of 12 C atoms in a 12-gram sample have determined.
 Source: inchcalculator.com
Source: inchcalculator.com
Add up all and assign unit as gramsmole. Molecular Weight To Moles - 9 images - molar mass molecular weight of n2o youtube how to convert grams to moles video lesson transcript. Therefore the mole is a unit for that physical quantity. Therefore the units of molar mass are gramsmoleHow to find the molar mass of a compound. The mole is the SI unit of the measurement for the amount of a substance.
 Source: clutchprep.com
Source: clutchprep.com
Lets figure out what the difference between molar mass and atomic mass is and learn to use molar mass as a conversion factor and stop guessing on how to con. The mole is the SI unit of the measurement for the amount of a substance. U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da. So in our example carbon has a molar mass of 1201 grams per mole. Use this page to learn how to convert between moles Glycerol and gram.
 Source: youtube.com
Source: youtube.com
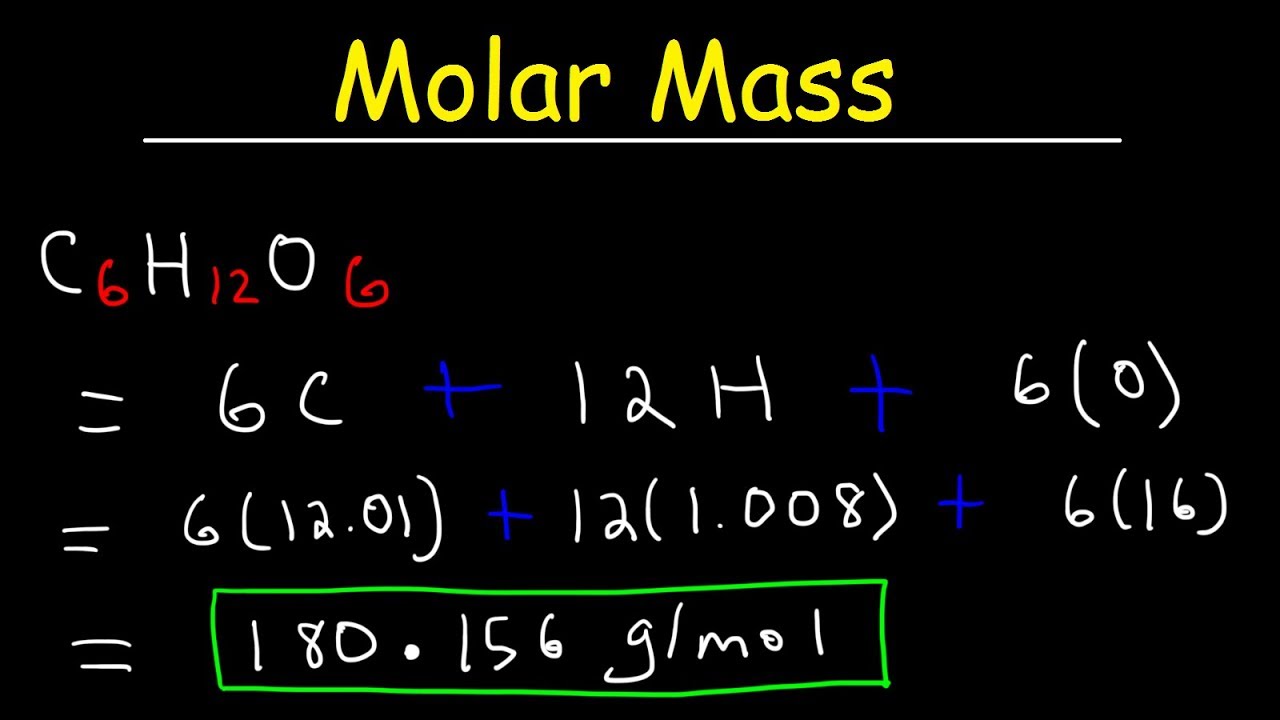
1201076 1007944 159994 100794 120107 1599942 100794. Moles to Grams Conversion Formula Questions. The molar mass of a substance is the mass in grams of 1 mole of the substance. Convert grams Salicylic Acid to moles or moles Salicylic Acid to grams. Therefore the molecular mass of H2SO4 is.
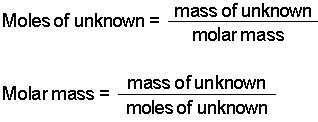 Source: chem.purdue.edu
Source: chem.purdue.edu
Always multiply the subscript by the atomic mass and when its done where it equals 3198 grams. Gram per mole gmol 1. But wait what actually is a mole. Use this page to learn how to convert between moles Glycerol and gram. So the molar mass for AgNO₃ is ____ grams.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to find molar mass in grams per mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






