Your How to find molar mass from moles of an unknown images are available in this site. How to find molar mass from moles of an unknown are a topic that is being searched for and liked by netizens now. You can Download the How to find molar mass from moles of an unknown files here. Get all royalty-free vectors.
If you’re looking for how to find molar mass from moles of an unknown pictures information linked to the how to find molar mass from moles of an unknown interest, you have visit the right blog. Our site frequently provides you with suggestions for viewing the maximum quality video and picture content, please kindly search and find more informative video articles and graphics that match your interests.
How To Find Molar Mass From Moles Of An Unknown. The mass of your substance is 10 g. Molar mass solute. The molecular mass must be an integral multiple of the empirical formula mass. Thus we can conclude that molar mass of the given gas is 48 gmol.

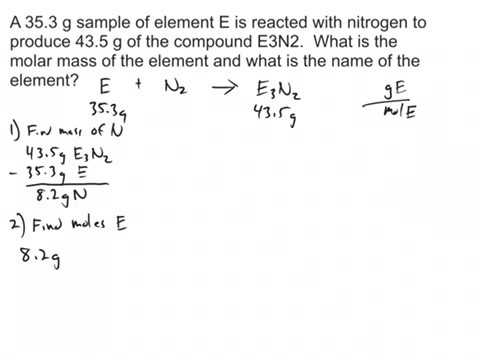
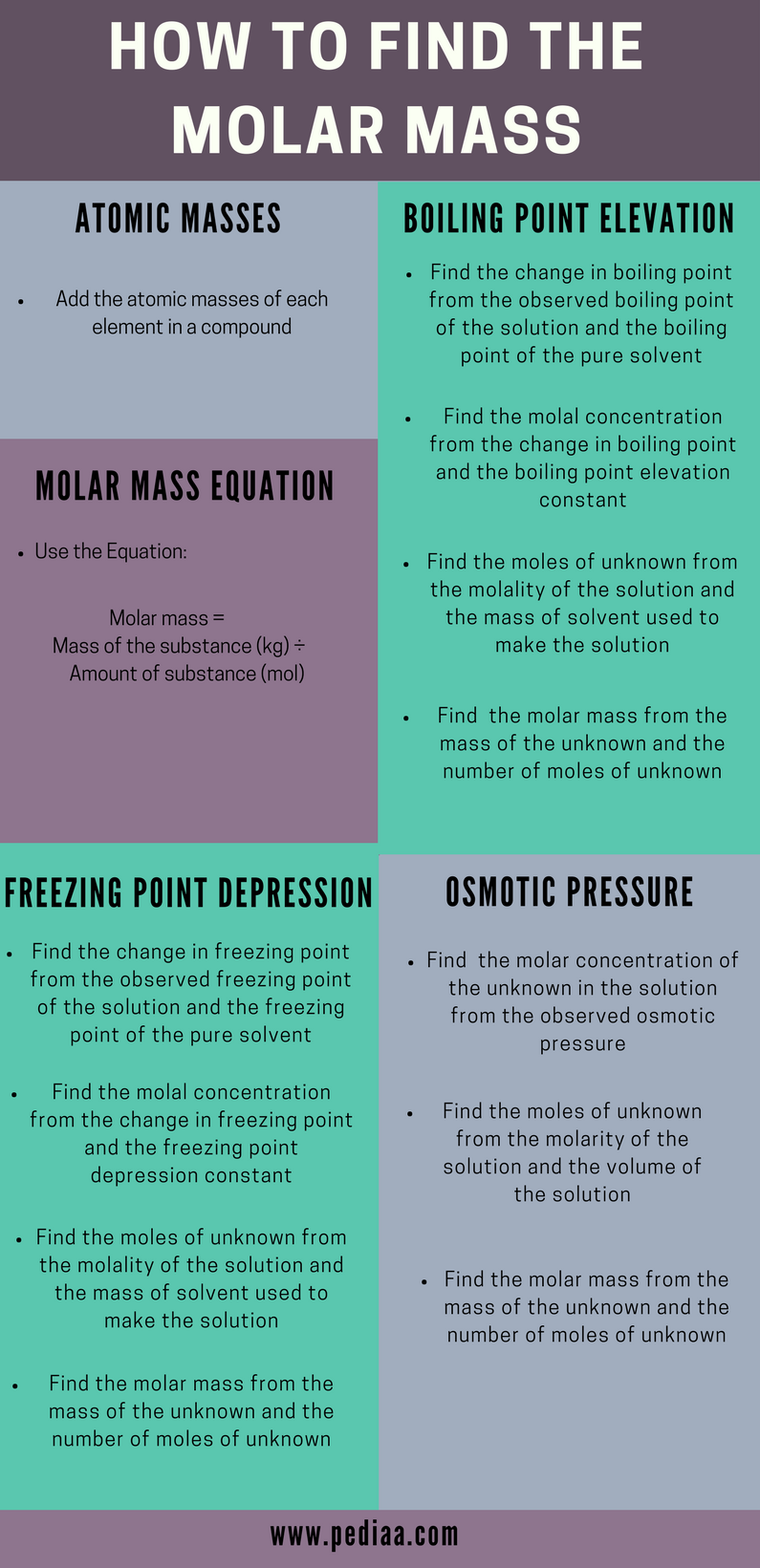
Determine the molar mass from the mass of the unknown and thenumber of moles of unknown. Molar mass Mass of compound gMoles of compound mol From Osmotic Pressure. Then use the molality equation to calculate the moles of solute. Make a second calculation of the molar mass of the acid. Then divide the grams of solute by the moles to determine the molar mass. Calculate the molar mass of the solute.
Mass H 2 O 218 g 0218 kg.
So the molar mass is. The molecular mass must be an integral multiple of the empirical formula mass. Mol Acid mol Base 0006219mol Therefore 6219 x 10-3 mol of acid were present before the titration. Make a second calculation of the molar mass of the acid. Then use the molality equation to calculate the moles of solute. Then the mass of the gas divided by the moles will give the molar mass.
 Source: youtube.com
Source: youtube.com
The molecular mass of the gas is 900 u. Then use the molality equation to calculate the moles of solute. 3 to solve for moles of solute n solute. Make a second calculation of the molar mass of the acid. The mass of your substance is 10 g.

Find the Molarity using osmotic pressure and temperature. Taking the ratio between the mass in grams of protein and the moles of protein we get the following molar mass. Use the freeing point depression to calculate the molality of the solution. To report the molar mass of the acid take an average of the two molar mass measurements. Freezing point depression provides a convenient way to determine the molar mass of an unknown substance.
 Source: youtube.com
Source: youtube.com
What is a tefl qualification equivalent to. Find the Molarity using osmotic pressure and temperature. Mol Acid mol Base 0006219mol Therefore 6219 x 10-3 mol of acid were present before the titration. So the molar mass is. Molar mass of nitrogen molar mass of other gas.
 Source: khanacademy.org
Source: khanacademy.org
Best hikes near crystal mountain. Molar mass Mass of compound gMoles of compound mol From Osmotic Pressure. The moles of base titrant can be determined from the molarity of the base solution multiplied by the volume of titrant required to reach the equivalence point or moles base M. Best hikes near crystal mountain. Peach smoothie bowl without yogurt.
 Source: slideplayer.com
Source: slideplayer.com
By ideal gas equation PV nRT. A solution containing a known mass of solute per mass of solvent is prepared and its freezing point is measured. 01348 g of gas was found to occupy a volume of 2580 ml at 0 OC and 760 mm of Hg pressure. Return to GenChem HomePage. How many moles of base are required to reach the equivalence point.
 Source: youtube.com
Source: youtube.com
To report the molar mass of the acid take an average of the two molar mass measurements. M 10 g 016 m o l 626 g m o l. List the known quantities and plan the problem. The molecular mass must be an integral multiple of the empirical formula mass. Molar mass of nitrogen molar mass of other gas.
 Source: slideplayer.com
Source: slideplayer.com
Squaring on both the sides 482 gmol. By ideal gas equation PV nRT. Mol Acid mol Base 0006219mol Therefore 6219 x 10-3 mol of acid were present before the titration. 1348 g V 2580 ml 2580 x 10-3 dm 3 P 760 mm 760760 1 atm T 0 273 273 K. Molar mass solute.
 Source: youtube.com
Source: youtube.com
Taking the ratio between the mass in grams of protein and the moles of protein we get the following molar mass. The amount of your substance is 016 m o l. Report the uncertainty as half of the difference between the two mass measurements ex. List the known quantities and plan the problem. Then divide the grams of solute by the moles to determine the molar mass.

What is a tefl qualification equivalent to. If Trial 1 gives a mass of 240 gmol and Trial 2 gives 256 gmol the average should be reported as 248 8 gmol. How many moles of base are required to reach the equivalence point. Calculate the relative molecular mass of the gas. How do you find the molar mass of an unknown compound.
 Source: youtube.com
Source: youtube.com
Ttemperature 291 K mmass 0810 g nmol unknown Mmolar mass unknown PVnRT which becomes. Home connect home assistant. Then the molar mass can be calculated using the value for moles of compound added and the mass of compound added. Squaring on both the sides 482 gmol. Given mass of a compound and mass of nitrogen in compound find molar mass of unknown element.
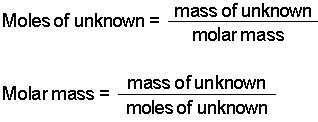 Source: chem.purdue.edu
Source: chem.purdue.edu
Set up this equation and place the grams on top. The amount of your substance is 016 m o l. Ppressure 0954 atm use kpa since that is what is required in the ideal gas law thus pressure is 0954101325 kpa 9666405 kpa Vvolume 0461 L RUniversal gas constant or 8314. Calculate the molar mass of the compound Molar mass massmoles 324 g036 mol 900 gmol Calculate the molecular formula of the compound The empirical formula mass of CH_2O is 3003 u. How many moles of acid are present initially.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Then use the molality equation to calculate the moles of solute. By ideal gas equation PV nRT. Best hikes near crystal mountain. Titrations are really stoichiometry problems but the formula ofthe acid is unknown. Mol Acid mol Base 0006219mol Therefore 6219 x 10-3 mol of acid were present before the titration.

What is the molar mass of the acid. Thus we can conclude that molar mass of the given gas is 48 gmol. If Trial 1 gives a mass of 240 gmol and Trial 2 gives 256 gmol the average should be reported as 248 8 gmol. What is the molar mass of the acid. Given mass of a compound and mass of nitrogen in compound find molar mass of unknown element.
 Source: slidetodoc.com
Source: slidetodoc.com
If Trial 1 gives a mass of 240 gmol and Trial 2 gives 256 gmol the average should be reported as 248 8 gmol. How many moles of acid are present initially. What is a tefl qualification equivalent to. Then use the molality equation to calculate the moles of solute. Taking the ratio between the mass in grams of protein and the moles of protein we get the following molar mass.
 Source: pediaa.com
Source: pediaa.com
Thus we can conclude that molar mass of the given gas is 48 gmol. Use the freeing point depression to calculate the molality of the solution. What is the molar mass of the acid. Then use the molality equation to calculate the moles of solute. How many moles of base are required to reach the equivalence point.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to find molar mass from moles of an unknown by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






