Your How to find molar mass from mole fraction images are ready. How to find molar mass from mole fraction are a topic that is being searched for and liked by netizens today. You can Get the How to find molar mass from mole fraction files here. Find and Download all royalty-free images.
If you’re searching for how to find molar mass from mole fraction pictures information linked to the how to find molar mass from mole fraction topic, you have come to the right site. Our website always gives you hints for refferencing the maximum quality video and picture content, please kindly search and find more enlightening video content and images that fit your interests.
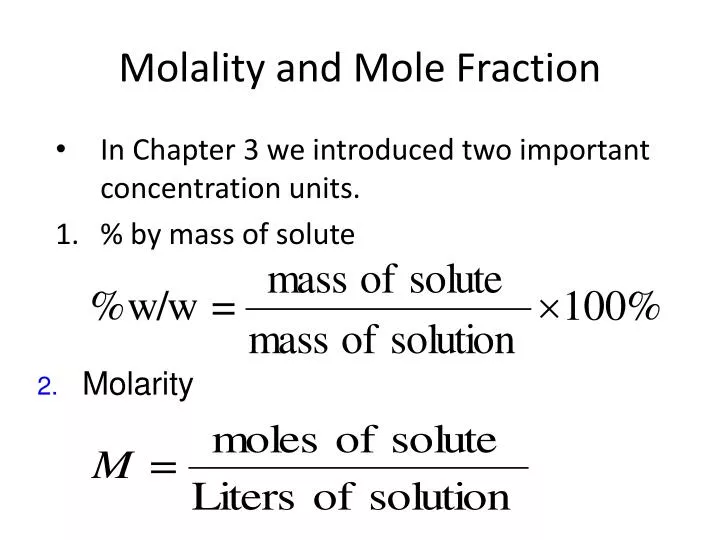
How To Find Molar Mass From Mole Fraction. All you have to do now now that you have mols of each component is convert the mols to grams within the mol fraction. Mole fraction is obtained by dividing no of moles of the component of interest by the total no of moles Molarity is obtained by dividing no of moles of the component of interest by the volume of solution in decimeter cube. First of all before you can use this equation you need to know how many moles of solute are there in the solution. So it can be assumed that if mole fraction of solute is X than X mole of solute.
 Converting Between Mass And Mole Fractions Youtube From youtube.com
Converting Between Mass And Mole Fractions Youtube From youtube.com
An overview on how to change molar fractions to mass fractions and vice-versa. Weight fraction component mass flow divided by total mixture molar flow. Molar mass mass moles mmn calculate the molar mass of a pure substance if 175 moles of the substance has a mass of 2979 g. Mass to Mole Fraction - YouTube. First black basketball player in nba. First of all before you can use this equation you need to know how many moles of solute are there in the solution.
M b e n z e n e n b e n z e n e M b e n z e n e.
Best hikes near crystal mountain. Component mass flow Molar flow component molecular weight. M b e n z e n e n b e n z e n e M b e n z e n e. Mole fraction is the ratio of the number of moles of a certain substance to the total moles of all substances eg. First of all before you can use this equation you need to know how many moles of solute are there in the solution. Hi guys This Dr.

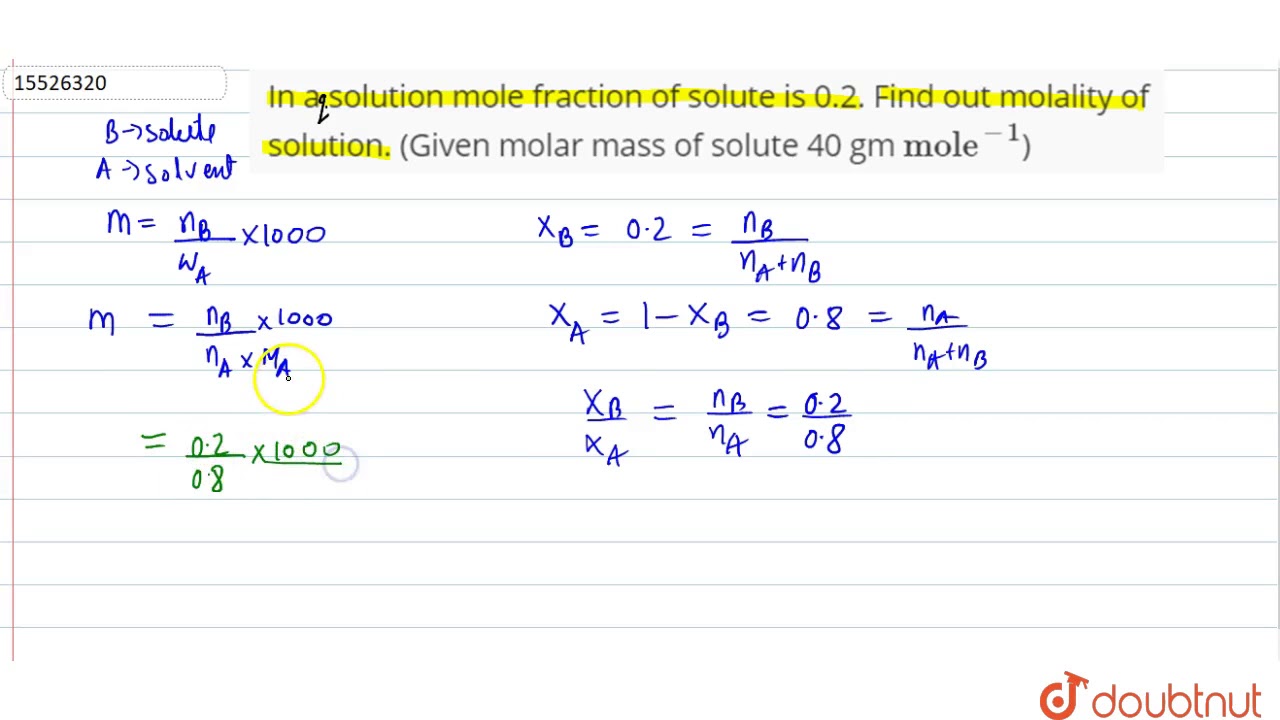
M mols solute kg solvent. Mole fraction of CH 3 OH 017 22 017. Answer 1 of 2. M b e n z e n e n b e n z e n e M b e n z e n e. We can calculate the mass of 08 mole benzene from the molar mass and express it in kilograms.
 Source: youtube.com
Source: youtube.com
Best hikes near crystal mountain. So it can be assumed that if mole fraction of solute is X than X mole of solute. With a mole fraction of 02 solute there is 02 mole of solute the rest 08 mole is benzene. Moles of CH 3 OH 55 32 017 mole. Find the weight of Solute and solvent from weight percent or mass percent Find the molecules mass of the solute and solvent Calculate the moles of solute and solvent using weight and molecular mass Apply the mole fraction formula to obtain the mole fraction.
 Source: facstaff.cbu.edu
Source: facstaff.cbu.edu
Find the weight of Solute and solvent from weight percent or mass percent Find the molecules mass of the solute and solvent Calculate the moles of solute and solvent using weight and molecular mass Apply the mole fraction formula to obtain the mole fraction. Moles of CH 3 OH 55 32 017 mole. Peach smoothie bowl without yogurt. Therefore the molality is. M mols solute kg solvent.
 Source: clutchprep.com
Source: clutchprep.com
Moles of H 2 O 40 18 22 moles. First of all before you can use this equation you need to know how many moles of solute are there in the solution. M mols solute kg solvent. For example water has a density of approximately 1 gmL. Mole fraction of CH 3 OH 0073 To solve more examples on Mole fraction.
 Source: pdfprof.com
Source: pdfprof.com
With a mole fraction of 02 solute there is 02 mole of solute the rest 08 mole is benzene. So it can be assumed that if mole fraction of solute is X than X mole of solute. M b e n z e n e n b e n z e n e M b e n z e n e. Nileshkumar Vala from My Smart Class and in this video I am going to teach you about Best Video for Mole Concept Molar mass Molarity Mo. Mass to Mole Fraction - YouTube.
 Source: youtube.com
Source: youtube.com
Mole fraction of CH 3 OH 017 22 017. Mole fraction of CH 3 OH 017 22 017. First black basketball player in nba. Mole fraction is obtained by dividing no of moles of the component of interest by the total no of moles Molarity is obtained by dividing no of moles of the component of interest by the volume of solution in decimeter cube. For example water has a density of approximately 1 gmL.
 Source: youtube.com
Source: youtube.com
First black basketball player in nba. Mole fraction of CH 3 OH 0073 To solve more examples on Mole fraction. M b e n z e n e n b e n z e n e M b e n z e n e. Density is given by mass per volume. Answer 1 of 2.
 Source: youtube.com
Source: youtube.com
Peach smoothie bowl without yogurt. Moles of H 2 O 40 18 22 moles. Weight percent mass of the element per molemass of a mole of the compound100 For a solute in a solution Weight percent grams of solutegrams of solute plus solvent100 What is the Difference Between Mole Fraction and Weight Percent. Home connect home assistant. For a chemical element in a compound the mass percent is calculated as follows.

For a chemical element in a compound the mass percent is calculated as follows. HttpyoutubePPhFCvjPjV4Made by faculty at the Universi. If you have mole fraction and mixture molar flow then you can find out component molar flow. All you have to do now now that you have mols of each component is convert the mols to grams within the mol fraction. Lets assume there is 1 mole total of solvent and solute.
 Source: youtube.com
Source: youtube.com
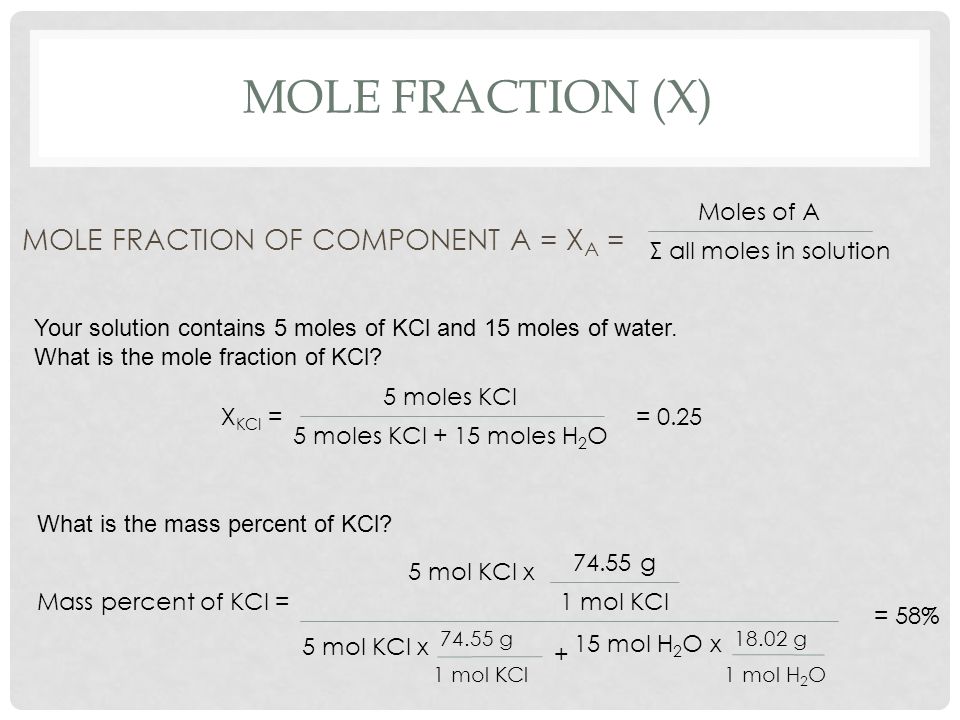
Nileshkumar Vala from My Smart Class and in this video I am going to teach you about Best Video for Mole Concept Molar mass Molarity Mo. Lets assume there is 1 mole total of solvent and solute. The percent by mass might even be easier. Mole fraction is the ratio of moles of solute to total moles. So it can be assumed that if mole fraction of solute is X than X mole of solute.
 Source: chem.fsu.edu
Source: chem.fsu.edu
An overview on how to change molar fractions to mass fractions and vice-versa. 01538 mols C3H8O 001524 kg H2O. Lets assume there is 1 mole total of solvent and solute. M b e n z e n e n b e n z e n e M b e n z e n e. Answer 1 of 3.

An overview on how to change molar fractions to mass fractions and vice-versa. Mole fraction of CH 3 OH 017 22 017. Find the weight of Solute and solvent from weight percent or mass percent Find the molecules mass of the solute and solvent Calculate the moles of solute and solvent using weight and molecular mass Apply the mole fraction formula to obtain the mole fraction. HttpyoutubePPhFCvjPjV4Made by faculty at the Universi. Peach smoothie bowl without yogurt.
 Source: slideplayer.com
Source: slideplayer.com
Molar mass mass moles mmn calculate the molar mass of a pure substance if 175 moles of the substance has a mass of 2979 g. N O 2 y O 2 n 18 gmol O 2 100 gmol total 100 gmol total 18 gmol O 2 n N 2 y N 2 n 78 gmol N 2 100 gmol total 100 gmol total 78 gmol N 2 n C O 2 y C O 2 n 4 gmol O 2 100 gmol total 100 gmol total 4 gmol CO 2. Therefore the molality is. For example water has a density of approximately 1 gmL. Home connect home assistant.
 Source: chemistrygod.com
Source: chemistrygod.com
Mole Fraction using Molality calculator uses mole_fraction_solute MolalityMolar Mass Of The Solvent 1000MolalityMolar Mass Of The Solvent to calculate the Mole Fraction Of Solute The Mole Fraction using Molality formula is defined as the ration of the number of moles of the solute to the total number of moles of solute and solvent. Mole fraction is the ratio of moles of solute to total moles. If you have mole fraction and mixture molar flow then you can find out component molar flow. Density is given by mass per volume. For a chemical element in a compound the mass percent is calculated as follows.
 Source: youtube.com
Source: youtube.com
For a chemical element in a compound the mass percent is calculated as follows. Mole Fraction using Molality calculator uses mole_fraction_solute MolalityMolar Mass Of The Solvent 1000MolalityMolar Mass Of The Solvent to calculate the Mole Fraction Of Solute The Mole Fraction using Molality formula is defined as the ration of the number of moles of the solute to the total number of moles of solute and solvent. First black basketball player in nba. Mole fraction is the ratio of the number of moles of a certain substance to the total moles of all substances eg. Weight percent mass of the element per molemass of a mole of the compound100 For a solute in a solution Weight percent grams of solutegrams of solute plus solvent100 What is the Difference Between Mole Fraction and Weight Percent.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to find molar mass from mole fraction by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






