Your How to find molar mass from grams and volume images are available in this site. How to find molar mass from grams and volume are a topic that is being searched for and liked by netizens today. You can Get the How to find molar mass from grams and volume files here. Find and Download all free vectors.
If you’re looking for how to find molar mass from grams and volume images information connected with to the how to find molar mass from grams and volume keyword, you have visit the right site. Our site frequently provides you with suggestions for seeking the maximum quality video and picture content, please kindly surf and locate more informative video articles and graphics that match your interests.
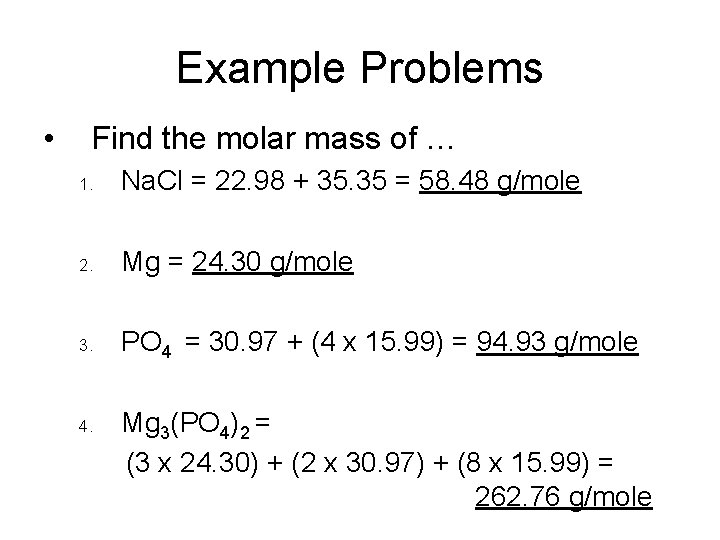
How To Find Molar Mass From Grams And Volume. You can use Molar mass of the substance alone to calculate molar mass. Multiply the molar mass by the number of moles to get the grams. Molarity x molar mass 3. To find the molar mass find the atomic mass of all the components of a chemical.
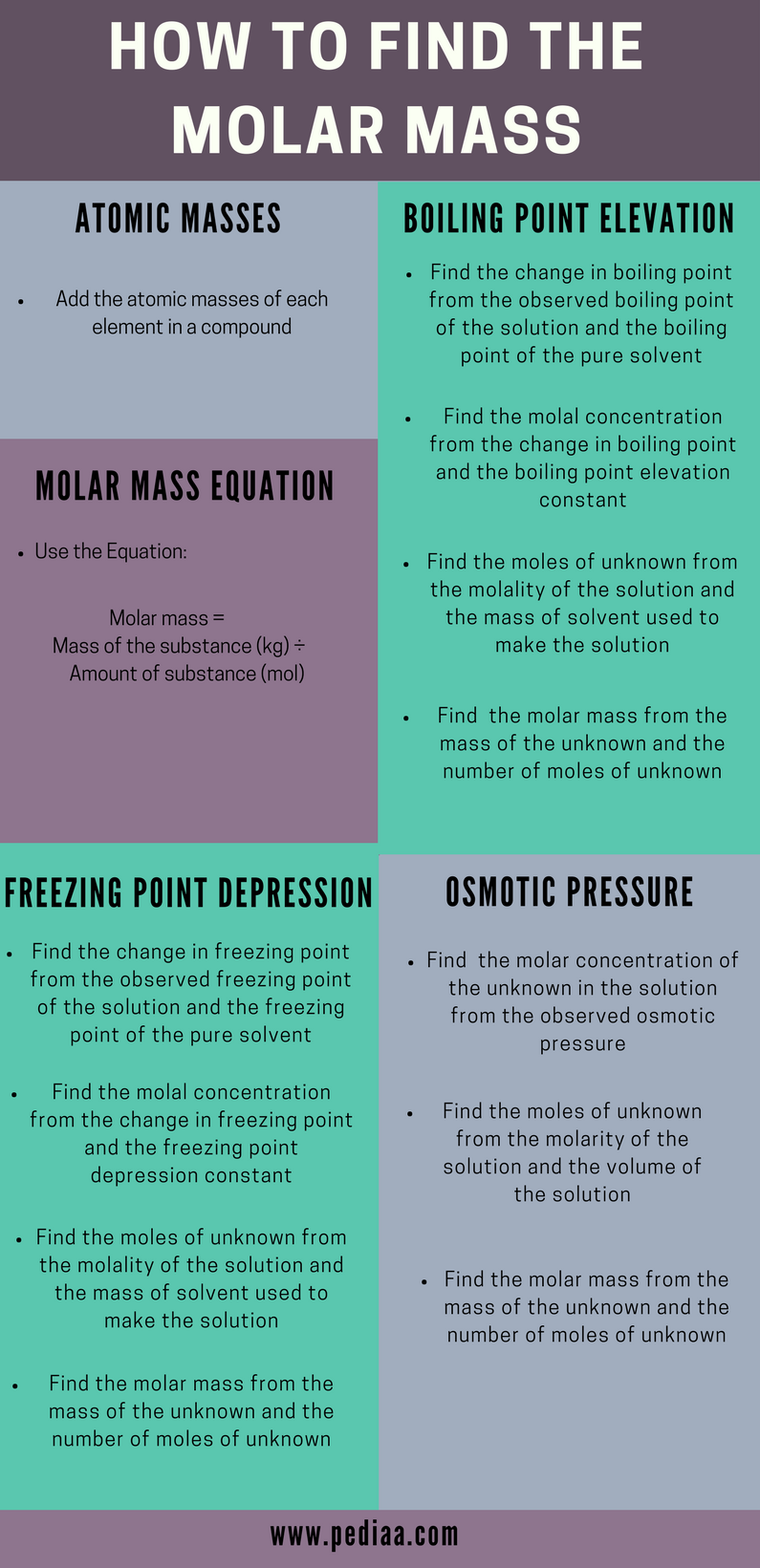 How To Find Molar Mass Different Methods Of Calculation Explained With Examples From pediaa.com
How To Find Molar Mass Different Methods Of Calculation Explained With Examples From pediaa.com
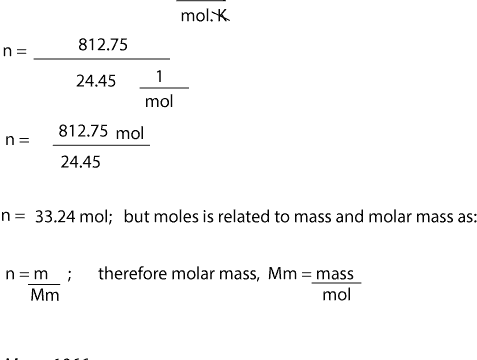
Mass g Concentration molL Volume L Formula Weight gmol What is mass. Once we get the value for moles we can then divide the mass of gas by moles to get the molar mass. P 1 atm. N - the number of moles of gas. N massMolar mass n 0025180 n 0000139 mol Please tell me this works now. Molar mass is equal to density in gL multiplied by molar volume.
N massMolar mass n 0025180 n 0000139 mol Please tell me this works now.
We can therefore write that n m M which can be used in the ideal gas law equation to get the value of the gas molar mass. Multiply the molar mass by the number of moles to get the grams. V 10 dm 3 t 14 C T 14 273 287 K P 729 mm of Hg W 17925 g. Calculate the amount of the solute for 1 L. What is formula weight. According to the equation the ratio is 13.
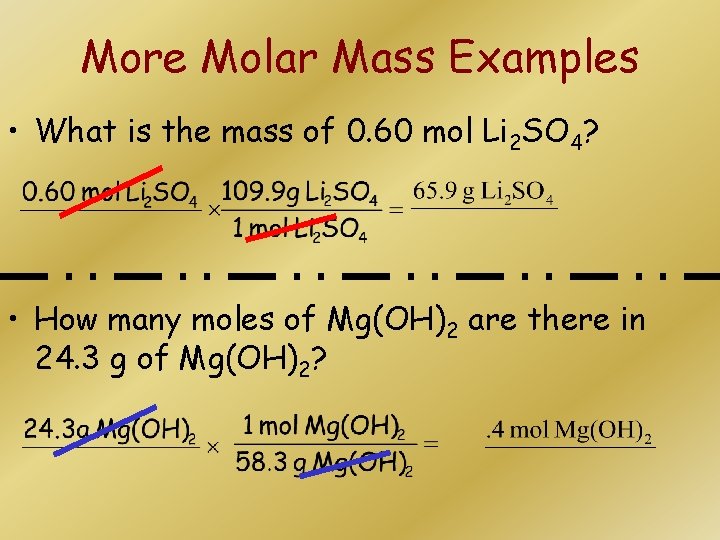 Source: slidetodoc.com
Source: slidetodoc.com
ρ 3165gL so m 3165 x 224 71896g. P V m M RT M mRT P V so. M ρV and m is the molar mass if V is the molar volume 224L Example. What is formula weight. Find out the molar mass of the substance hint.
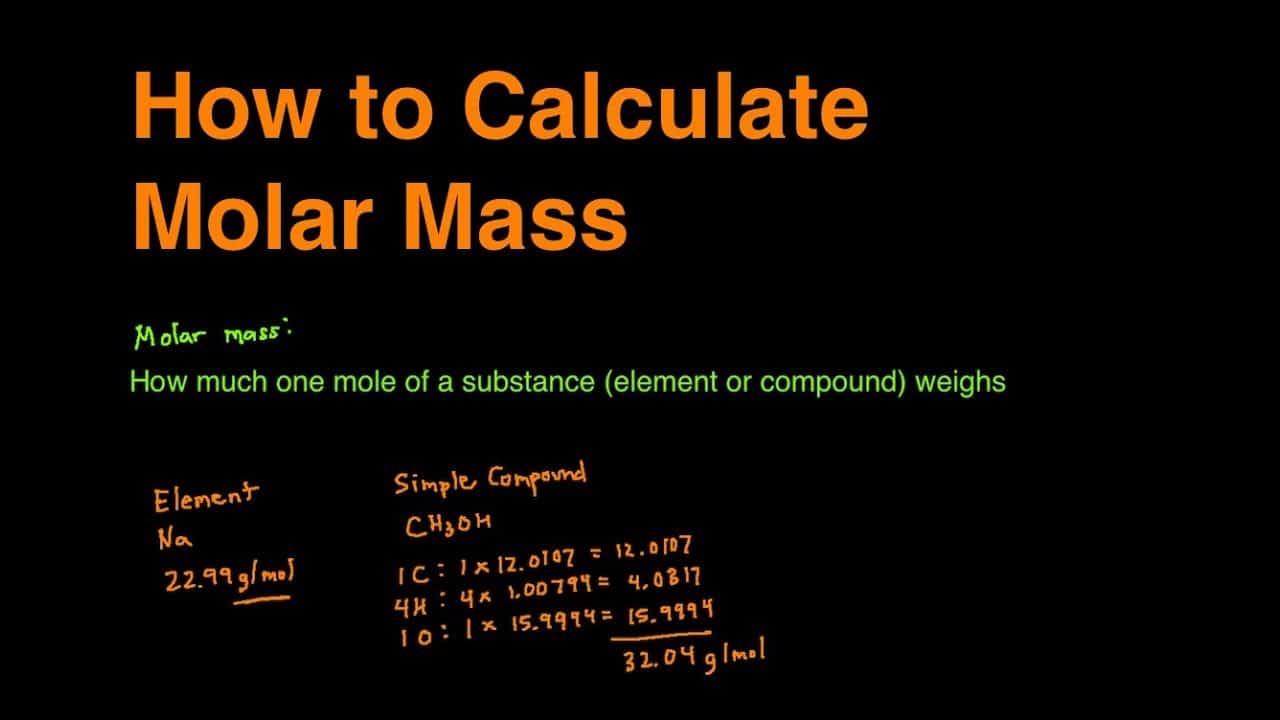 Source: geteducationcrunch.com
Source: geteducationcrunch.com
The molar mass of KClO3 is 122548 gmol. Recall that the ideal gas equation is given as. To find the molar mass find the atomic mass of all the components of a chemical. M m R T P V M 21 g 62364 L m m H g m o l K 80 27313 K 120 m m H g 15 L M 257 g m o l. The Glucose is 0000139 mol and the Phenylhydrazine hydrochloride is 00028 mol.

M m R T P V M 21 g 62364 L m m H g m o l K 80 27313 K 120 m m H g 15 L M 257 g m o l. M 132 186 07097 moles per kg of water 07097 110 1000 moles per 110 ml of water 00780 moles these molese have a mass of 200 g so molar mass 200 00780 256 g mol. MCO2 12011 gmol C 215999 gmol O 44009 g CO2mol CO2. Calculate the amount of the solute for 1 L. M ρV and m is the molar mass if V is the molar volume 224L Example.
 Source: youtube.com
Source: youtube.com
The molar mass of KClO3 is 122548 gmol. M ρV and m is the molar mass if V is the molar volume 224L Example. Calculate the relative molecular mass of the gas. 01348 g of gas was found to occupy a volume of 2580 ml at 0 OC and 760 mm of Hg pressure. Mass m is the amount of matter present in a substance.
 Source: youtube.com
Source: youtube.com
Recall that the ideal gas equation is given as. MCO2 12011 gmol C 215999 gmol O 44009 g CO2mol CO2. If we know mass pressure volume and temperature of a gas we can calculate its molar mass by using the ideal gas equation. M 132 186 07097 moles per kg of water 07097 110 1000 moles per 110 ml of water 00780 moles these molese have a mass of 200 g so molar mass 200 00780 256 g mol. P V m M RT M mRT P V so.
 Source: omnicalculator.com
Source: omnicalculator.com
M m R T P V M 21 g 62364 L m m H g m o l K 80 27313 K 120 m m H g 15 L M 257 g m o l. N - the number of moles of gas. 01348 g of gas was found to occupy a volume of 2580 ml at 0 OC and 760 mm of Hg pressure. N massMolar mass n 0025180 n 0000139 mol Please tell me this works now. M m RT P V.
 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
M m n where. M 132 186 07097 moles per kg of water 07097 110 1000 moles per 110 ml of water 00780 moles these molese have a mass of 200 g so molar mass 200 00780 256 g mol. M m R T P V M 21 g 62364 L m m H g m o l K 80 27313 K 120 m m H g 15 L M 257 g m o l. M m RT P V. Find the molar mass of chlorine Cl2.
 Source: slideserve.com
Source: slideserve.com
1348 g V 2580 ml 2580 x 10 -3 dm 3 P 760 mm 760760 1 atm T 0 273 273 K. There are 23811 grams of hydrogen peroxide in 0700 moles of hydrogen peroxide. N - the number of moles of gas. To prepare 1 L of 05 M sodium chloride solution then as per the formula use 2922 g of sodium chloride 05 molL 1L 5844 gmol. Find the molar mass of a water molecule h₂o.
 Source: geteducationcrunch.com
Source: geteducationcrunch.com
Solve for m at STP. You will be using the molar mass of water frequently so it is a good idea to memorize it as 1802 gmol When calculating the molar mass of a compound it is important to remember what each numerical subscript is indicating. Glucose molar mass 180 gmol So can I now find n. The number of grams of KClO3 will be 30637. Mass m is the amount of matter present in a substance.
 Source: slidetodoc.com
Source: slidetodoc.com
N massMolar mass n 0025180 n 0000139 mol Please tell me this works now. We can therefore write that n m M which can be used in the ideal gas law equation to get the value of the gas molar mass. You need to know the molar mass of the solute. Calculate the amount of the solute for 1 L. M ρV and m is the molar mass if V is the molar volume 224L Example.
 Source: youtube.com
Source: youtube.com
Grams of hydrogen peroxide 34016 gramsmol x 0700 mol 23811 grams. The Glucose is 0000139 mol and the Phenylhydrazine hydrochloride is 00028 mol. Recall that the ideal gas equation is given as. Calculate the relative molecular mass of the gas. V 10 dm 3 t 14 C T 14 273 287 K P 729 mm of Hg W 17925 g.
 Source: youtube.com
Source: youtube.com
M ρV and m is the molar mass if V is the molar volume 224L Example. You can use Molar mass of the substance alone to calculate molar mass. Calculate the molar mass of the solute. In the first problem practice determining either moles volume or molarity from the two that are given using variations of equation 1. Volume L Mass g Concentration molL x Molecular Weight gmol.
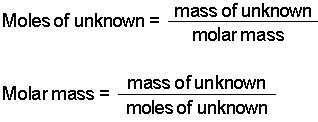 Source: chem.purdue.edu
Source: chem.purdue.edu
Calculate the amount of the solute for 1 L. Hereof is density equal to molar mass. Find the molar mass of the gas. Mass molar concentration volume and formula weight are related to each other as follows. According to the equation the ratio is 13.
 Source: pediaa.com
Source: pediaa.com
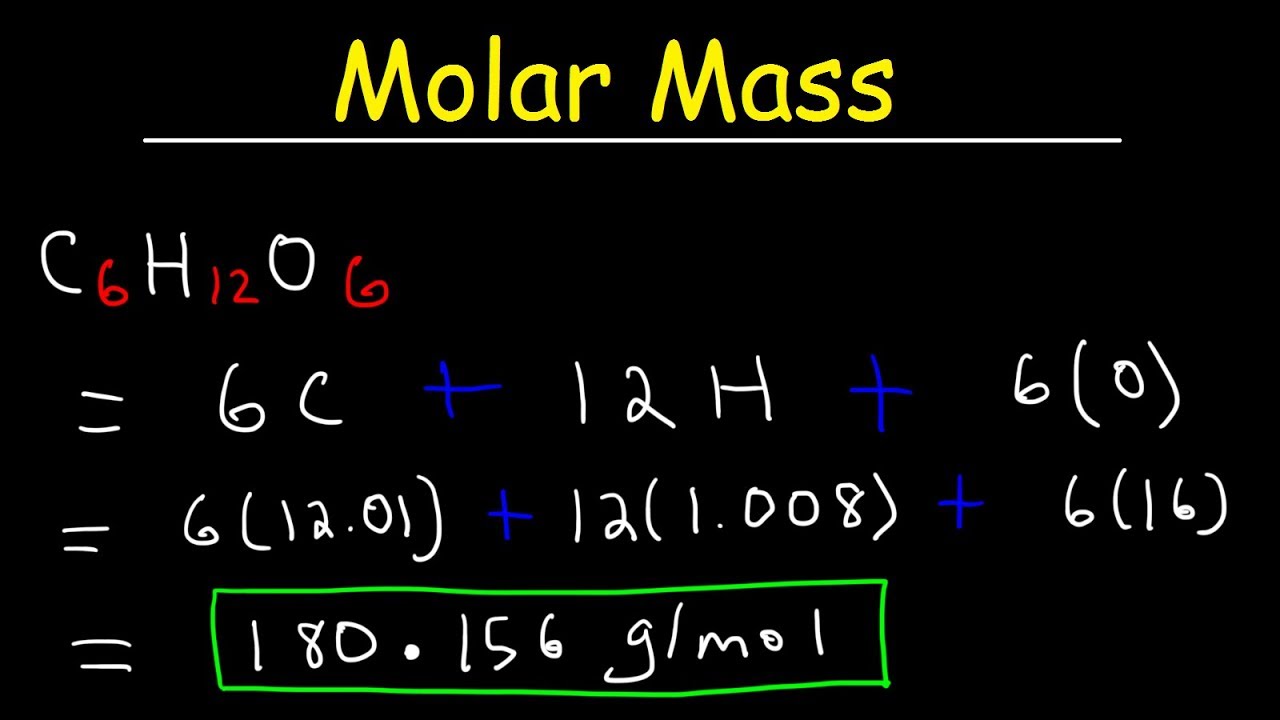
Molar mass 34016 gramsmol. N massMolar mass n 0025180 n 0000139 mol Please tell me this works now. The molar mass of H 2 O is 1802 gmol. Find out the molar mass of the substance hint. Find the molar mass of the gas.
 Source: youtube.com
Source: youtube.com
Molar concentration is the amount of a solute present in one unit of a solution. Use the ideal gas law to calculate mass of CO2. Molar concentration is the amount of a solute present in one unit of a solution. Heres an example of how this would look in a problem. The molar mass of KClO3 is 122548 gmol.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to find molar mass from grams and volume by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






