Your How to find grams per mole of a compound images are ready. How to find grams per mole of a compound are a topic that is being searched for and liked by netizens today. You can Find and Download the How to find grams per mole of a compound files here. Get all royalty-free images.
If you’re looking for how to find grams per mole of a compound pictures information connected with to the how to find grams per mole of a compound topic, you have pay a visit to the right blog. Our site frequently provides you with hints for downloading the maximum quality video and image content, please kindly search and find more informative video content and images that fit your interests.
How To Find Grams Per Mole Of A Compound. Numerically this would be 2 1008 1 1600 18016. Converting grams to moles involves 2 steps. In order to determine the number of moles of a given compound the first thing you need to do is find the molecular mass or molecular weight of the compound in question. Start by looking up the number of grams per mole of potassium and chlorine on a periodic table.
 How To Convert Grams To Moles Video Lesson Transcript Study Com From study.com
How To Convert Grams To Moles Video Lesson Transcript Study Com From study.com
The molar mass of a compound sometimes to referred to as molecular weight is the cumulative atomic weight of all the atomselements in the compound. Numerically this would be 2 1008 1 1600 18016. 26 Votes This is the weight in grams of 1 mole of the compound. Multiply both the values. For example 25 grams of water equals 2518016 or 139 moles. This will give you the relative amount that each element contributes to the.
It has units of grams per mole.
Determine the mass in grams of each element in the sample. Calculate the number of milligrams required for the chemical reaction by multiplying the number millimoles by the weight of one millimole of the compound. The number of milligrams per millimole is equivalent to the number of grams per mole. This compound is also known as Magnesium Chloride. Divide the number of grams of the compound by its molecular mass. The answer is 0010502988100114We assume you are converting between moles MgCl2 and gram.
 Source: hu.pinterest.com
Source: hu.pinterest.com
The answer is 0010502988100114We assume you are converting between moles MgCl2 and gram. Then add them together to get the grams per mole for KCl. If you know the quantity of mole it can be converted into grams and vice versa. One mole consists of Avogadro number of atoms. Use the chemical formula to determine the number of each type of atom present in the compound.
 Source: khanacademy.org
Source: khanacademy.org
The number of milligrams per millimole is equivalent to the number of grams per mole. Start by looking up the number of grams per mole of potassium and chlorine on a periodic table. Then add them together to get the grams per mole for KCl. Then add all of your answers together to find the molar mass of the compound. N m M where M is the molar mass of this material.
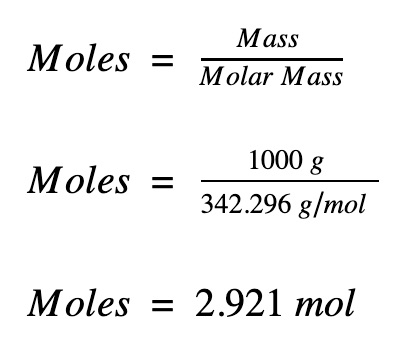 Source: surfguppy.com
Source: surfguppy.com
N m M where M is the molar mass of this material. Cl 355 gmol. Then add all of your answers together to find the molar mass of the compound. One mole consists of Avogadro number of atoms. Usually the units used for this are grams per moleIn this movie we show how to calculate the molecular weight of a substance from the atomic weights given on the periodic table.
 Source: pinterest.com
Source: pinterest.com
485 14507 Views. And it gives the conversion factor of 1 585. KCl 391 355 746 gmol. Multiply the atomic weight from the periodic table of each element by the number of atoms of that element present in the compound. N m M where M is the molar mass of this material.
 Source: pinterest.com
Source: pinterest.com
The units for molar mass are therefore gramsmole. Calculating Molecular Weight Molar MassThe molecular weight is the mass of one mole of a substance. This is the molar mass of the compound. And it gives the conversion factor of 1 585. 1 mole is equal to 1 moles MgCl2 or 95211 grams.
 Source: pinterest.com
Source: pinterest.com
This is the molar mass of the compound. The answer is the number of moles of that mass of compound. It has units of grams per mole. Click to see full answer. So for every one mole of glucose C6H12O6 we have 18016 grams of glucose C6H12O6 and this is going to get us we get 152 times 1000 is equal to this is the number of grams of glucose we have and then were going to divide by 18016 divide by 18016 gives us this number and lets see if we see significant figures we have three significant figures here we have five here so.
 Source: pinterest.com
Source: pinterest.com
26 Votes This is the weight in grams of 1 mole of the compound. To find the molar mass of a compound. For example the molar mass of oxygen is 16 grams and one millimole of oxygen contains 0016 grams or 16 milligrams. 26 Votes This is the weight in grams of 1 mole of the compound. N m M where M is the molar mass of this material.
 Source: pinterest.com
Source: pinterest.com
For example a molecule has a molecular weight of 18018 gmol. N m M where M is the molar mass of this material. Example 1 Calculate the mass in grams of 36 mol of H2SO4. Percent Element in a Compound Percent compositions of elements are calculated by using molecular formula of compound. This is the molar mass of the compound.
 Source: youtube.com
Source: youtube.com
1 millimole Fe2O3 15969 1000 01597 grams 15969 milligrams. Cl 355 gmol. Determine the mass in grams of each element in the sample. The mole the unit of measurement for amount of substance is defined in such a way that the molar mass of a compound in gmol is numerically equal to the average mass of one molecule in. Converting grams to moles involves 2 steps.
 Source: study.com
Source: study.com
N m M where M is the molar mass of this material. To find the molar mass of a compound. Divide the mass of the compound in grams by the molar mass you just calculated. Multiply the atomic weight from the periodic table of each element by the number of atoms of that element present in the compound. Divide the number of grams of the compound by its molecular mass.
 Source: pinterest.com
Source: pinterest.com
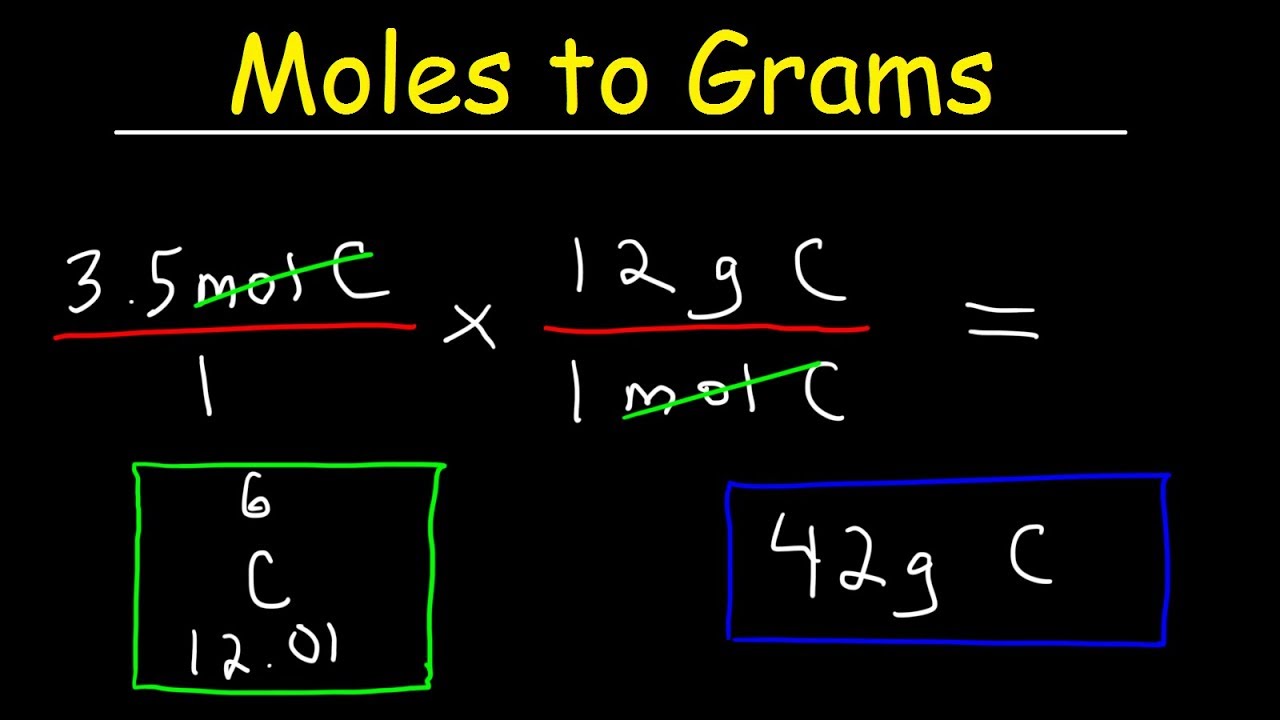
Multiply both the values. To find the molar mass of a compound. A millimole is one thousandth of that number and it likewise contains one thousandth of a moles mass. Use the chemical formula to determine the number of each type of atom present in the compound. 485 14507 Views.
 Source: youtube.com
Source: youtube.com
1 millimole Fe2O3 15969 1000 01597 grams 15969 milligrams. Multiply both the values. Example 1 Calculate the mass in grams of 36 mol of H2SO4. The molar mass is the mass of one mole of a. 1 mole 746 g 3 grams 3 746 0040 moles Express this as moles per kilogram solution.
 Source: pinterest.com
Source: pinterest.com
A millimole is one thousandth of that number and it likewise contains one thousandth of a moles mass. The mole the unit of measurement for amount of substance is defined in such a way that the molar mass of a compound in gmol is numerically equal to the average mass of one molecule in. This compound is also known as Magnesium Chloride. So for every one mole of glucose C6H12O6 we have 18016 grams of glucose C6H12O6 and this is going to get us we get 152 times 1000 is equal to this is the number of grams of glucose we have and then were going to divide by 18016 divide by 18016 gives us this number and lets see if we see significant figures we have three significant figures here we have five here so. The total is the molecular weight of the compound.
 Source: in.pinterest.com
Source: in.pinterest.com
The total is the molecular weight of the compound. 1 millimole Fe2O3 15969 1000 01597 grams 15969 milligrams. It is found to contain 4000 carbon 672 hydrogen and. The units for molar mass are therefore gramsmole. Now multiply 25 g.
 Source: pinterest.com
Source: pinterest.com
Finally divide the number of grams of the compound by the molar mass of the compound to find the number of moles. And it gives the conversion factor of 1 585. Cl 355 gmol. If you know the quantity of mole it can be converted into grams and vice versa. 485 14507 Views.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find grams per mole of a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






