Your How to find grams per liter from molarity images are ready in this website. How to find grams per liter from molarity are a topic that is being searched for and liked by netizens now. You can Download the How to find grams per liter from molarity files here. Find and Download all free photos and vectors.
If you’re searching for how to find grams per liter from molarity pictures information connected with to the how to find grams per liter from molarity interest, you have visit the right blog. Our site frequently provides you with suggestions for seeing the highest quality video and picture content, please kindly hunt and find more enlightening video articles and images that fit your interests.
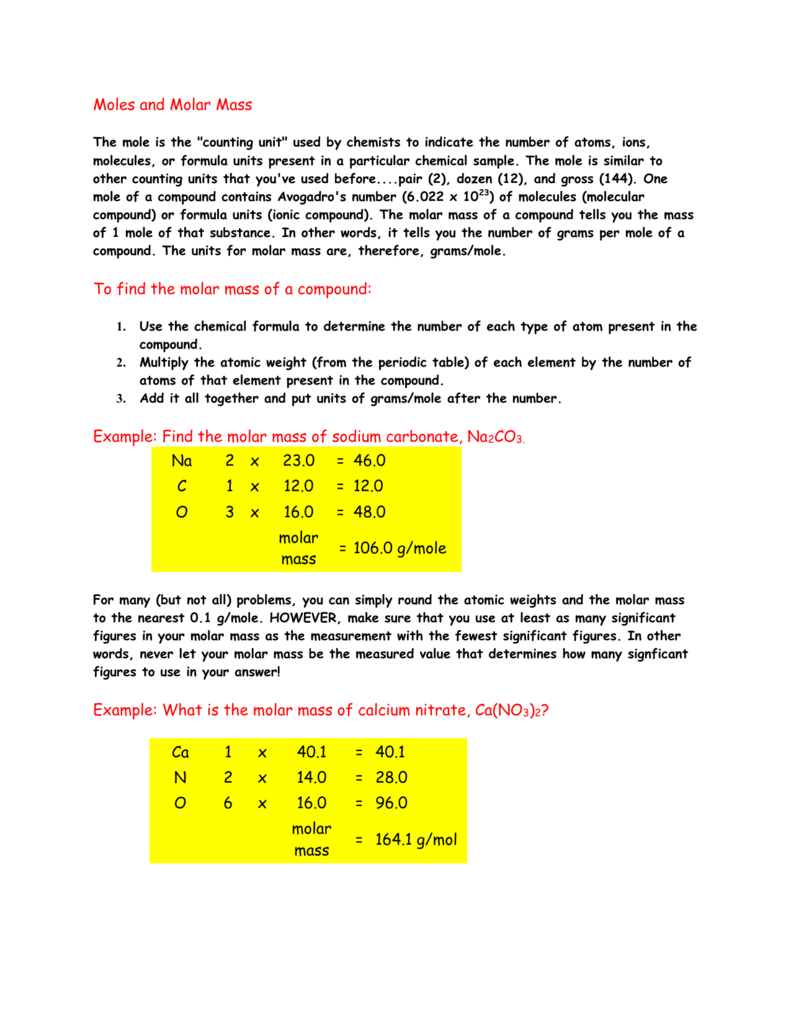
How To Find Grams Per Liter From Molarity. Students know how to calculate the concentration of a solute in terms of grams per liter molarity parts per million and percent composition. For NaCl the molar mass is 5844 gmol. Most of your household chemicals are solutions. M is equivalent to 1 mole of substance in a liter of solution.
 Toluene Formula And Structure Toluene Formula Molar Mass From in.pinterest.com
Toluene Formula And Structure Toluene Formula Molar Mass From in.pinterest.com
For NaCl the molar mass is 5844 gmol. This is enough to calculate the molarity. This measure of concentration is most often used when discussing the solubility of a solid in solution. Molarity M is defined as the number of moles of solute n divided by the volume V of the solution in liters. Therefore Gm per liter Molarity Molecular mass. Of gram molesVolume Mass of substance in gmMolecular massVolume.
Mass g No.
Liters of solution mL of solution x 1 L1000 mL Liters of solution 750 mL x 1 L1000 mL Liters of solution 075 L. M is equivalent to 1 mole of substance in a liter of solution. Convert gL to mgL. GL grams per liter mass of solute volume of solution F formality formula weight units per liter of solution ppm parts per million ratio of parts of solute per 1 million parts of the solution. To do this measure called molarity is commonly used. Therefore Gm per liter Molarity Molecular mass.
 Source: in.pinterest.com
Source: in.pinterest.com
Here a more favorable calculation for normality is considered where the normality of a solution is equal to the molarity multiplied by the number of equivalents. Molarity 015 moles of KMnO 4 075 L of solution. This is enough to calculate the molarity. This measure of concentration is most often used when discussing the solubility of a solid in solution. Molarity is the measure of concentration of a solution expressed as the number of moles of solute substance per a liter of solution.
 Source: in.pinterest.com
Source: in.pinterest.com
You can convert molarity into grams per liter by multiplying it with the molecular Molar mass ie. Liters of solution mL of solution x 1 L1000 mL Liters of solution 750 mL x 1 L1000 mL Liters of solution 075 L. Most of your household chemicals are solutions. Convert gL to mgL. Molarity 020 M.
 Source: in.pinterest.com
Source: in.pinterest.com
Molarity 015 moles of KMnO 4 075 L of solution. GL grams per liter mass of solute volume of solution F formality. Now we can use the rearranged equation. Molarity 015 moles of KMnO 4 075 L of solution. Here a more favorable calculation for normality is considered where the normality of a solution is equal to the molarity multiplied by the number of equivalents.
 Source: in.pinterest.com
Source: in.pinterest.com
1 liter 1000 milliliter Answer 705 gramliter x 11000 litermilliliter 00705 grammilliliter 705 gram per liter 00705 gram per milliliter. Molarity moles soluteLiter solution. Calculate the concentration in moles of solute per liter of solution of each of the following. Molarity is MolL which when multiplied with the Molecular Mass sometimes also called Molecular Weight becomes gramsliter. Multiply this by the molecular mass of the solute in grams per mol gmol.
 Source: pinterest.com
Source: pinterest.com
Convert gL to mgL. For NaCl the molar mass is 5844 gmol. Mass g Concentration molL x Volume L x Molecular Weight gmol Background. Then molar concentration mole liter and with the known. Mass g Concentration molL x Volume L x Molecular Weight gmol.
 Source: in.pinterest.com
Source: in.pinterest.com
Convert 750 mL to liters. Then molar concentration mole liter and with the known. It is important to note that the molarity is defined as moles of solute per liter of. Total number of moles 1 mole 585 g 6 g 062 moles Now determine moles per liter of solution. Of gram molesVolume Mass of substance in gmMolecular massVolume.
 Source: pinterest.com
Source: pinterest.com
Mass g Concentration molL x Volume L x Molecular Weight gmol Background. You can convert molarity into grams per liter by multiplying it with the molecular Molar mass ie. Moles mol Molarity. What is the equation to find grams per liter. It is important to note that the molarity is defined as moles of solute per liter of.
 Source: in.pinterest.com
Source: in.pinterest.com
Moles mol Molarity. Moles mol Molarity. Molarity moles soluteLiter solution. Total number of moles 1 mole 585 g 6 g 062 moles Now determine moles per liter of solution. Moles mol x Molar Mass.
 Source: in.pinterest.com
Source: in.pinterest.com
Students know how to calculate the concentration of a solute in terms of grams per liter molarity parts per million and percent composition. Therefore Gm per liter Molarity Molecular mass. Then by dividing the total number of moles by the total volume of the solution one can find the. This is enough to calculate the molarity. Molarity 015 moles of KMnO 4 075 L of solution.
 Source: in.pinterest.com
Source: in.pinterest.com
GL grams per liter mass of solute volume of solution F formality. Then by dividing the total number of moles by the total volume of the solution one can find the. Then molar concentration mole liter and with the known. Now we can use the rearranged equation. MolarityGm per moleMolecular mass.
 Source: in.pinterest.com
Source: in.pinterest.com
Then molar concentration mole liter and with the known. For example you may add salt to water when cooking pasta. This is enough to calculate the molarity. Mass g Concentration molL x Volume L x Molecular Weight gmol Background. This measure of concentration is most often used when discussing the solubility of a solid in solution.
 Source: in.pinterest.com
Source: in.pinterest.com
It is important to note that the molarity is defined as moles of solute per liter of. MolarityGm per moleMolecular mass. Most of your household chemicals are solutions. To convert mgmL to molarity requires converting milligrams to grams and then using the molar mass of the solute to find the total number of moles. Mass g Concentration molL x Volume L x Molecular Weight gmol.
 Source: pinterest.com
Source: pinterest.com
Then molar concentration mole liter and with the known. Then by dividing the total number of moles by the total volume of the solution one can find the. Mass g No. For example you may add salt to water when cooking pasta. M is equivalent to 1 mole of substance in a liter of solution.
 Source: in.pinterest.com
Source: in.pinterest.com
Grams per liter represent the mass of solute divided by the volume of solution in liters. Liters of solution mL of solution x 1 L1000 mL Liters of solution 750 mL x 1 L1000 mL Liters of solution 075 L. Multiply this by the molecular mass of the solute in grams per mol gmol. Most of your household chemicals are solutions. Molarity 015 moles of KMnO 4 075 L of solution.
 Source: pinterest.com
Source: pinterest.com
Mass g No. Students know how to calculate the concentration of a solute in terms of grams per liter molarity parts per million and percent composition. Molarity 015 moles of KMnO 4 075 L of solution. You can convert molarity into grams per liter by multiplying it with the molecular Molar mass ie. To convert mgmL to molarity requires converting milligrams to grams and then using the molar mass of the solute to find the total number of moles.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find grams per liter from molarity by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.