Your How to find grams of an element from moles of a compound images are ready. How to find grams of an element from moles of a compound are a topic that is being searched for and liked by netizens now. You can Get the How to find grams of an element from moles of a compound files here. Get all royalty-free vectors.
If you’re looking for how to find grams of an element from moles of a compound images information connected with to the how to find grams of an element from moles of a compound interest, you have pay a visit to the ideal blog. Our site frequently provides you with suggestions for viewing the highest quality video and picture content, please kindly surf and locate more enlightening video content and graphics that fit your interests.
How To Find Grams Of An Element From Moles Of A Compound. Moreover most of the molecules are made up of more than 1 element. I prompt the user if they would like to repeatyn then continue for the next element until the user is finished. Once youve done that you need to determine how much the compound weighs physically in grams. Divide Grams by Grams per Mole.
 Pin By Michelle M On Knowledge Molar Mass Teaching Chemistry Chemistry Lessons From pinterest.com
Pin By Michelle M On Knowledge Molar Mass Teaching Chemistry Chemistry Lessons From pinterest.com
However molar mass can also be calculated by multiplying the atomic mass in amu by the molar mass constant 1 gmol. Fifth convert to grams. Its all based on the definition. After you get both of these values you need to divide the physical weight of the compound by. First of all every element has a different molar mass and is expressed as gram per mole. May 5 2018 Heres how I do it.
Chemistry The Mole Concept The Mole.
Once youve done that you need to determine how much the compound weighs physically in grams. Basically I get the user to input Element Si Amount of element1 Element Weight28085. In order to determine the number of moles of a given compound the first thing you need to do is find the molecular mass or molecular weight of the compound in question. Calculate the mass in grams of each reactant. How to Calculate Moles in a Reaction Find Mass in Grams. For example lets take H X 2 element so nNNa – n Na N number of atoms 2 1grmol H 2 moles H x 5 moles in the compound 10 moles 10 moles H X 2 602 10 23 602 10 23 atoms Or do I do like this.
 Source: pinterest.com
Source: pinterest.com
Its all based on the definition. Divide Grams by Grams per Mole. Its all based on the definition. 1 If m is the mass of the compound in grams and M is the AMU mass of the compound there are N A m M molecules of the compound where N A is Avogadros number. For example lets take H X 2 element so nNNa – n Na N number of atoms 2 1grmol H 2 moles H x 5 moles in the compound 10 moles 10 moles H X 2 602 10 23 602 10 23 atoms Or do I do like this.
 Source: pinterest.com
Source: pinterest.com
Please explain about the way to calculate the number of atoms of a certain element in a given mass of a compound. The molar mass is the mass of a given chemical element or chemical compound g divided by the amount of substance mol. To calculate the molar mass of a compound with multiple atoms sum all the atomic mass of the constituent atoms. Apr 30 2018 Divide the mass of the compound in grams by the molar mass you just calculated. May 5 2018 Heres how I do it.
 Source: pinterest.com
Source: pinterest.com
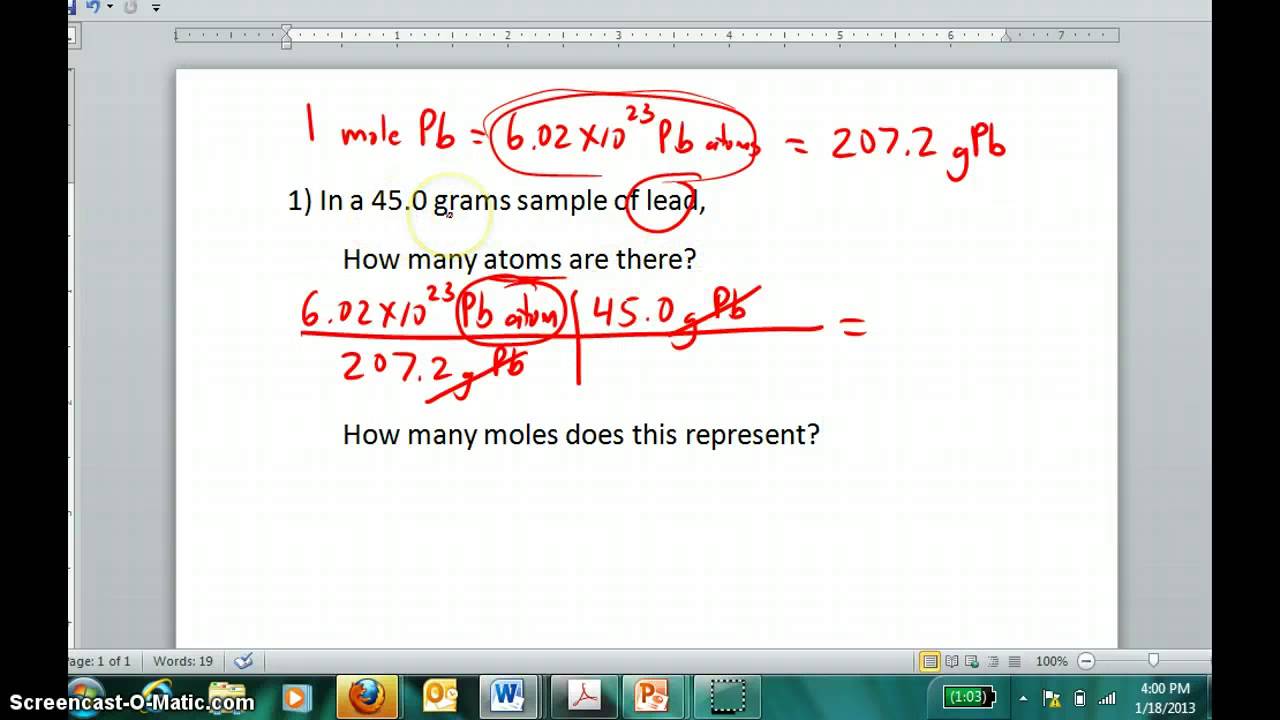
Converting between grams of a compound and grams of a constituent element Determine the mass of oxygen in a 58 g of NaHCO3 58 g NaCHO3 1 mol NaCHO3 8401 NaCHO3 4800 mol O 1 mol NaCHO3 1600 g O 1 mol I got 53 but the answer is 33 g O Help what am I doing wrong Monique Sep 23 2011. Chemistry The Mole Concept The Mole. How to Calculate Moles in a Reaction Find Mass in Grams. However molar mass can also be calculated by multiplying the atomic mass in amu by the molar mass constant 1 gmol. Determine the atomic weight of each element using the periodic table.
 Source: pinterest.com
Source: pinterest.com
The characteristic molar mass of an element is simply the atomic mass in gmol. Basically I get the user to input Element Si Amount of element1 Element Weight28085. 1 If m is the mass of the compound in grams and M is the AMU mass of the compound there are N A m M molecules of the compound where N A is Avogadros number. How to Find Moles. To find mass of 4 moles of carbon you multiple 4 number of molesby 6 molar mass giving you 24 grams.
 Source: pinterest.com
Source: pinterest.com
Calculate the mass in grams of each reactant. Its all based on the definition. Molar mass is the number of grams in one mole 602 x 10. The molar mass of a compound can be calculated by adding the standard atomic masses in gmol of the constituent atoms. In order to determine the number of moles of a given compound the first thing you need to do is find the molecular mass or molecular weight of the compound in question.
 Source: pinterest.com
Source: pinterest.com
0150 mol NH4 x 18042 1 mol 271g NH4 Converting 00301 moles into grams 00301 x 18011 mol 05421 g of NH4 ions. Molg sugar 1 18015588 mass sugar molg sugar 00055507486072616675. Moreover most of the molecules are made up of more than 1 element. To find mass of 4 moles of carbon you multiple 4 number of molesby 6 molar mass giving you 24 grams. For example lets take H X 2 element so nNNa – n Na N number of atoms 2 1grmol H 2 moles H x 5 moles in the compound 10 moles 10 moles H X 2 602 10 23 602 10 23 atoms Or do I do like this.
 Source: pinterest.com
Source: pinterest.com
Please explain about the way to calculate the number of atoms of a certain element in a given mass of a compound. Molar mass is the number of grams in one mole 602 x 10. How many atoms of each element are found in. First of all every element has a different molar mass and is expressed as gram per mole. To do this you must need to know its chemical formula which is C X 9 H X 13 N O X 3.
 Source: pinterest.com
Source: pinterest.com
5 moles H X 2 S O X 4 Now the only confusing thing for me is the 5 moles. Determine the atomic weight of each element using the periodic table. Molg sugar 1 18015588 mass sugar molg sugar 00055507486072616675. Molar mass is the number of grams in one mole 602 x 10. The mass of one mole of an element referred to as relative atomic mass in grams is the same as its mass number which you can see in the periodic table.
 Source: pinterest.com
Source: pinterest.com
After you get both of these values you need to divide the physical weight of the compound by. The percent by mass of a particular element in a chemical compound can be found by dividing the. How to Calculate Moles in a Reaction Find Mass in Grams. How do you find moles of any element. Its all based on the definition.
 Source: pinterest.com
Source: pinterest.com
To calculate the molar mass of a compound with multiple atoms sum all the atomic mass of the constituent atoms. The molar mass of a compound can be calculated by adding the standard atomic masses in gmol of the constituent atoms. However molar mass can also be calculated by multiplying the atomic mass in amu by the molar mass constant 1 gmol. Calculate the mass in grams of each reactant. Total mass of reactant O per gram sugar 1919928g total mass of reactant O per mol sugar 00055507486072616675 molg sugar.
 Source: pinterest.com
Source: pinterest.com
To find mass of 4 moles of carbon you multiple 4 number of molesby 6 molar mass giving you 24 grams. Fifth convert to grams. In order to determine the number of moles of a given compound the first thing you need to do is find the molecular mass or molecular weight of the compound in question. Total mass of reactant O per gram sugar 1919928g 00055507486072616675. Molg sugar 1 18015588 mass sugar molg sugar 00055507486072616675.
 Source: pinterest.com
Source: pinterest.com
Total mass of reactant O per gram sugar 1919928g total mass of reactant O per mol sugar 00055507486072616675 molg sugar. I prompt the user if they would like to repeatyn then continue for the next element until the user is finished. The mass of one mole of an element referred to as relative atomic mass in grams is the same as its mass number which you can see in the periodic table. Simply divide the mass with units of grams by the molar mass with units of grams PER mole And so if got a math440g math mass of mathCO_ 2 math we divide thru by the molar mass OF CARBON DIOXIDE. For hydrogen chloride the molar mass is 1007.
 Source: pinterest.com
Source: pinterest.com
How do you find moles of any element. Calculate Grams per Mole. The characteristic molar mass of an element is simply the atomic mass in gmol. For example the Chlorine Cl has a molar mass of 354530 gmol in the same way Sodium Na has a molar mass of 229898 gmol. However molar mass can also be calculated by multiplying the atomic mass in amu by the molar mass constant 1 gmol.
 Source: pinterest.com
Source: pinterest.com
First of all every element has a different molar mass and is expressed as gram per mole. First of all every element has a different molar mass and is expressed as gram per mole. Chemistry The Mole Concept The Mole. Moreover most of the molecules are made up of more than 1 element. May 5 2018 Heres how I do it.
 Source: pinterest.com
Source: pinterest.com
Once youve done that you need to determine how much the compound weighs physically in grams. Divide Grams by Grams per Mole. Total mass of reactant O per gram sugar 1919928g 00055507486072616675. I prompt the user if they would like to repeatyn then continue for the next element until the user is finished. After you get both of these values you need to divide the physical weight of the compound by.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to find grams of an element from moles of a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






