Your How to find grams if you have moles images are ready in this website. How to find grams if you have moles are a topic that is being searched for and liked by netizens today. You can Find and Download the How to find grams if you have moles files here. Download all royalty-free photos.
If you’re searching for how to find grams if you have moles pictures information linked to the how to find grams if you have moles keyword, you have pay a visit to the ideal site. Our website always gives you suggestions for seeking the highest quality video and image content, please kindly hunt and find more informative video content and images that match your interests.
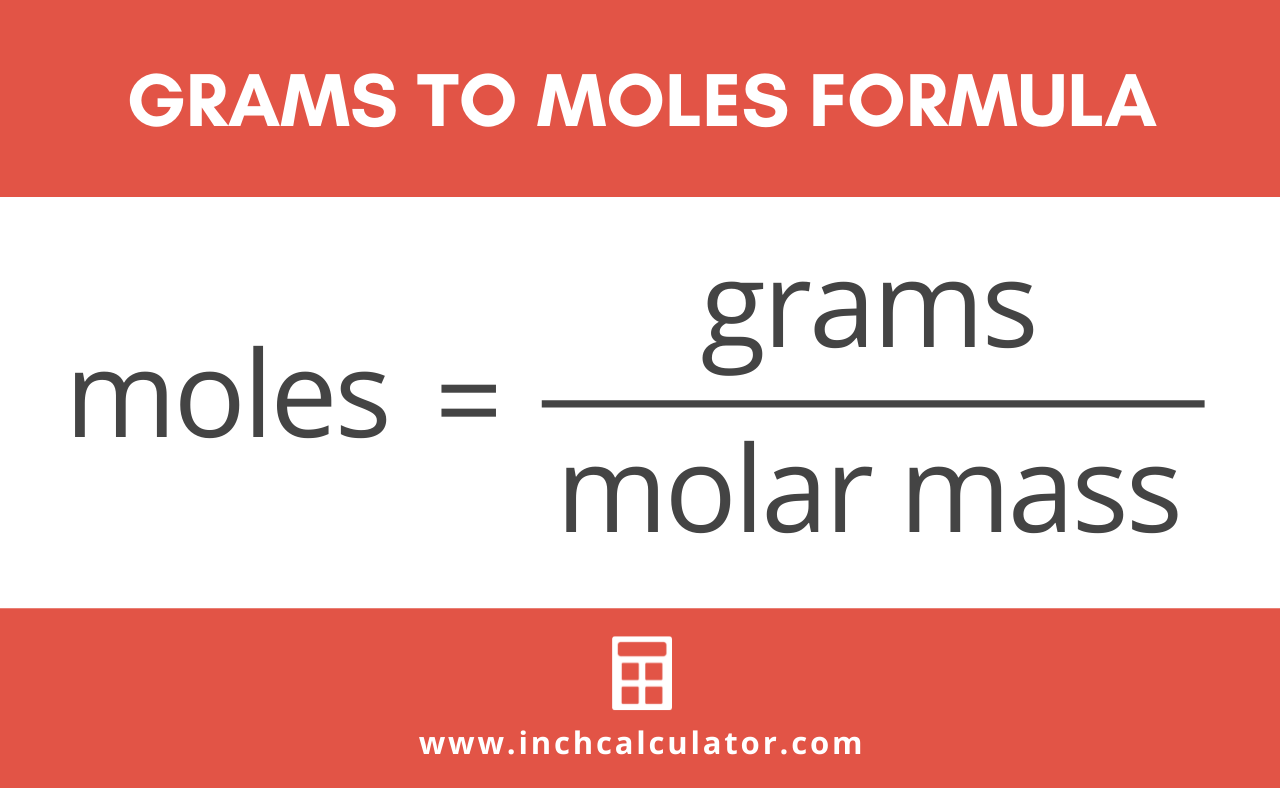
How To Find Grams If You Have Moles. More free chemistry help videos. The molecular formula for Phosphorus is P. Tips on how to convert from grams to moles for no matter scientific want you could have. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula.
 If Your Given Is In Moles Determine The Molar Ratio To Change From Moles Of Your Original Substance To Moles Of The Desired Substance If You Want Your Ppt Download From slideplayer.com
If Your Given Is In Moles Determine The Molar Ratio To Change From Moles Of Your Original Substance To Moles Of The Desired Substance If You Want Your Ppt Download From slideplayer.com
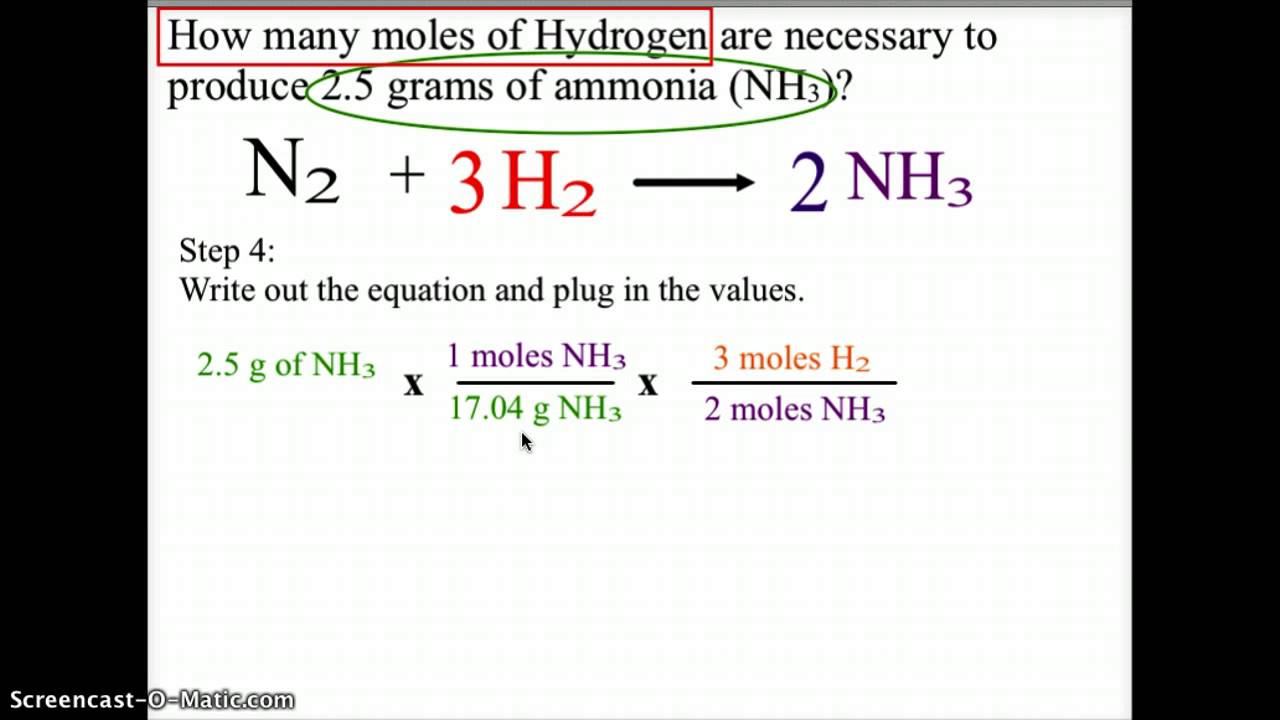
This chemistry video tutorial explains how to convert the unit grams to moles which is a common conversion step for many stoichiometry questions. The unit is typically gmol. When using this method always write 1 in front of the word moles 602 10 23 in front of the unit atoms or molecules and the molar mass in front of the unit grams If you dont your answer will be wrong. After doing so multiply the moles with the Avogadros number. 0700 mole x 340146 gramsmole 238 grams The answer of 238 g has been rounded to three significant figures because the 0700 value had the least number of significant figures in the problem. The molecular formula for Phosphorus is P.
For finding out this you have to multiply the mass of solute by its molar mass conversion factor.
1 week ago Moles to Grams Formula Equations - Definition Formula. Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4. Multiply both the values. Since moles grams of compoundthe molar mass to find the mass you will need to do mass molar mass x molarity. 170 mol 58443 g 1 mol 994 g. As an illustration suppose you have mixed 25 g of NaCl common salt into 2 litres of water then first you have to determine the numbers of moles in 25 g of NaCl Salt.
 Source: slideplayer.com
Source: slideplayer.com
After doing so multiply the moles with the Avogadros number. After doing so multiply the moles with the Avogadros number. 1 mole is equal to 1 moles Phosphorus or 30973761 grams. After about a semester and a half I had forgotten how to convert from grams to molesThis article was extremely clear precise and definitive. Look for the atomic masses of hydrogen sulfur and oxygen.
 Source: youtube.com
Source: youtube.com
To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. Find the molar mass of the substance. More free chemistry help videos. 600 g 58443 gmol 1027 mol of NaCl. 1 week ago Moles to Grams Formula Equations - Definition Formula.
 Source: clutchprep.com
Source: clutchprep.com
Multiply the moles given by the substances molar mass. Multiply the moles given by the substances molar mass. After about a semester and a half I had forgotten how to convert from grams to molesThis article was extremely clear precise and definitive. If you know the quantity of mole it can be converted into grams and vice versa. More free chemistry help videos.
 Source: khanacademy.org
Source: khanacademy.org
1 week ago Moles to Grams Formula Equations - Definition Formula. Thus the number of moles in the given sample of. Now we are already in litres so how to convert moles to grams. Multiply both the values. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance.
 Source: study.com
Source: study.com
Number of moles mass relative formula mass. On mole is made up of total number of Avogadro atoms and If you are sure of quantity of moles then they are converted to grams quickly. Note that rounding errors may occur so always check the results. The formula for moles to grams is given by. What do we multiply moleslitre by to get gramslitre.
 Source: youtube.com
Source: youtube.com
Molecular weight of Phosphorus or grams. In our example the weight of NaCl is 100 grams and its molecular weight is 5852 gmoles. You can also use our best moles grams calculator to determine the molecules or atoms present in grams. Look for the atomic masses of hydrogen sulfur and oxygen. When using this method always write 1 in front of the word moles 602 10 23 in front of the unit atoms or molecules and the molar mass in front of the unit grams If you dont your answer will be wrong.
 Source: youtube.com
Source: youtube.com
For finding out this you have to multiply the mass of solute by its molar mass conversion factor. Finding molar mass starts with units of grams per mole gmol. Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4. 0700 mole x 340146 gramsmole 238 grams The answer of 238 g has been rounded to three significant figures because the 0700 value had the least number of significant figures in the problem. It can also be.
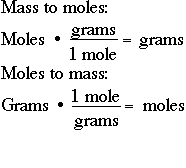 Source: socratic.org
Source: socratic.org
To go from moles to grams multiply by the formula mass. Number of moles Weight of compound in grams molecular weight of compound. 4 If this problem were set up like the proportion above you would have this. Multiply the variety of moles by the molar mass to acquire the ultimate reply in grams. Now we are already in litres so how to convert moles to grams.
 Source: wikihow.com
Source: wikihow.com
When using this method always write 1 in front of the word moles 602 10 23 in front of the unit atoms or molecules and the molar mass in front of the unit grams If you dont your answer will be wrong. Number of moles mass relative formula mass. You can also use our best moles grams calculator to determine the molecules or atoms present in grams. The formula for moles to grams is given by. You can view more details on each measurement unit.
 Source: inchcalculator.com
Source: inchcalculator.com
For example if you have 170 mol of NaCl then. This chemistry video tutorial explains how to convert the unit grams to moles which is a common conversion step for many stoichiometry questions. This can be rearranged to find the mass if the number of moles and molar mass its relative formula mass in grams are known. As an illustration suppose you have mixed 25 g of NaCl common salt into 2 litres of water then first you have to determine the numbers of moles in 25 g of NaCl Salt. Thus the number of moles in the given sample of.
 Source: youtube.com
Source: youtube.com
1 mole is equal to 1 moles Phosphorus or 30973761 grams. Multiply the moles given by the substances molar mass. Find the molar mass of the substance. Number of moles mass relative formula mass. How to Convert Grams to Moles.
 Source: youtube.com
Source: youtube.com
For example if you have 600 g of NaCl then. Since water has two molecules of hydrogen and one molecule of oxygen then the molecular weight of water is 1801528gmol. The formula for moles to grams is given by Grams Moles times AtomicWeight. This can be rearranged to find the mass if the number of moles and molar mass its relative formula mass in grams are known. Look for the atomic masses of hydrogen sulfur and oxygen.
 Source: slideplayer.com
Source: slideplayer.com
It is equal to Avogadros number NA namely 6022 x10 23If we have one mole of water then we know that it will have a mass of 2 grams for 2 moles of H atoms 16 grams for one mole O atom 18 grams. To go from moles to grams multiply by the formula mass. Tips on how to convert from grams to moles for no matter scientific want you could have. What do we multiply moleslitre by to get gramslitre. It is equal to Avogadros number NA namely 6022 x10 23If we have one mole of water then we know that it will have a mass of 2 grams for 2 moles of H atoms 16 grams for one mole O atom 18 grams.
 Source: slideplayer.com
Source: slideplayer.com
It is equal to Avogadros number NA namely 6022 x10 23If we have one mole of water then we know that it will have a mass of 2 grams for 2 moles of H atoms 16 grams for one mole O atom 18 grams. For finding out this you have to multiply the mass of solute by its molar mass conversion factor. What do we multiply moleslitre by to get gramslitre. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. 170 mol 58443 g 1 mol 994 g.
 Source: study.com
Source: study.com
1 day ago One mole consists of Avogadro number of atomsIf you know the quantity of mole it can be. N m M where M is the molar mass of this material. Multiply the variety of moles by the molar mass to acquire the ultimate reply in grams. The unit is typically gmol. Multiply the moles given by the substances molar mass.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find grams if you have moles by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






