Your How to find grams from mol images are ready. How to find grams from mol are a topic that is being searched for and liked by netizens now. You can Download the How to find grams from mol files here. Find and Download all royalty-free vectors.
If you’re searching for how to find grams from mol pictures information linked to the how to find grams from mol topic, you have pay a visit to the ideal site. Our site always gives you suggestions for downloading the highest quality video and picture content, please kindly hunt and find more informative video articles and graphics that match your interests.
How To Find Grams From Mol. You can view more details on each measurement unit. 1 grams H2O is equal to 0. How many grams H2O in 1 mol. Finding molar mass starts with units of grams per mole gmol.
 How To Convert Between Molecules Moles And Grams Examples Practice Problems Summary Youtube From youtube.com
How To Convert Between Molecules Moles And Grams Examples Practice Problems Summary Youtube From youtube.com
How many grams H2O in 1 mol. You can view more details on each measurement unit. For a given molecule one mole is a mass in grams whose number is equal to the molar mass of the molecule. Molecular weight of H2O or mol This compound is also known as Water or Dihydrogen Monoxide. Finding molar mass starts with units of grams per mole gmol. The answer is 1801528.
For example the water molecule has an molar mass of 18 therefore one mole of water weighs 18 grams.
Molecular weight of H2O or mol This compound is also known as Water or Dihydrogen Monoxide. The SI base unit for amount of substance is the mole. Using the chemical formula of the compound and the periodic table of elements we can add up the atomic weights and calculate molecular weight of the substance. This site explains how to find molar mass. The answer is 1801528. One lb-mol is equal to 45359237 mol which value is the same as the number of grams in an international avoirdupois pound.
 Source: slideplayer.com
Source: slideplayer.com
We assume you are converting between grams H2O and mole. Using the chemical formula of the compound and the periodic table of elements we can add up the atomic weights and calculate molecular weight of the substance. We assume you are converting between grams H2O and mole. In the metric system chemical engineers once used the kilogram-mole notation kg-mol which is defined as the number of entities in 12 kg of 12 C and often referred to the mole as the gram-mole notation g-mol when. You can view more details on each measurement unit.
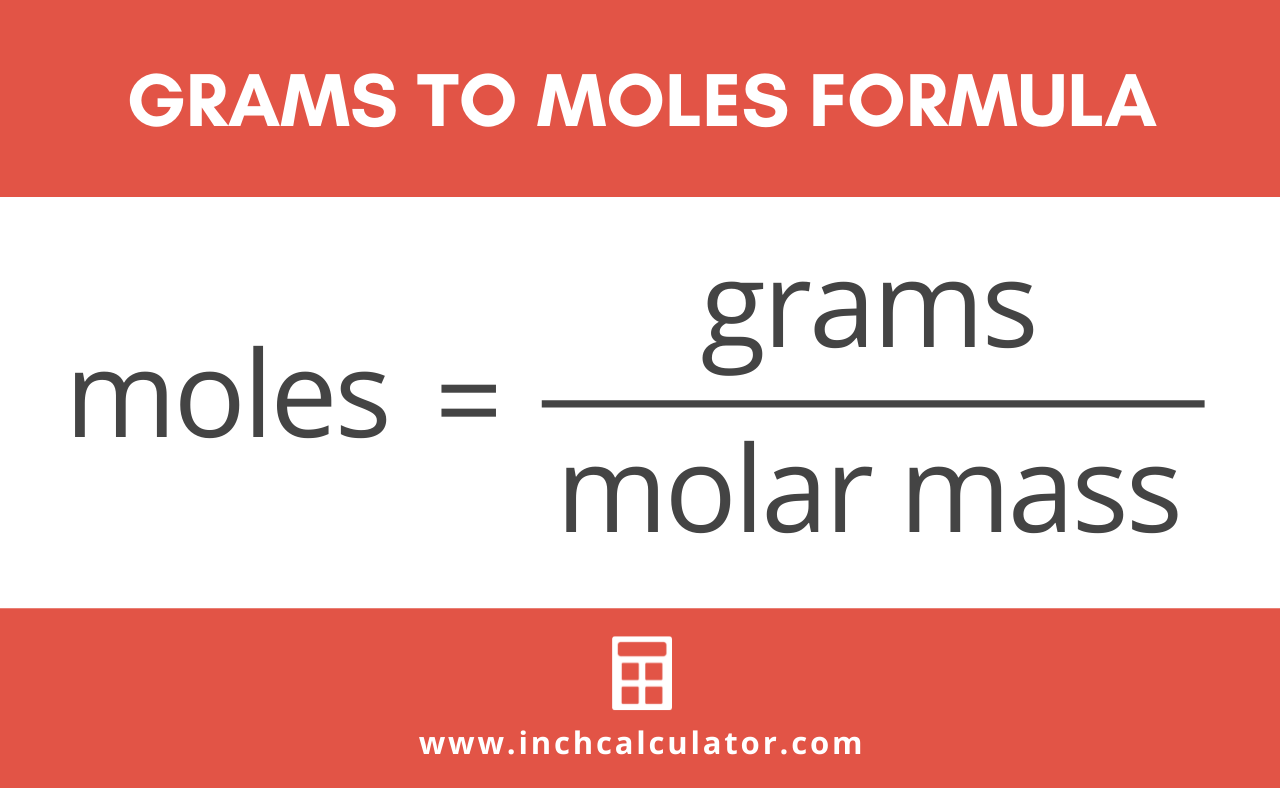 Source: inchcalculator.com
Source: inchcalculator.com
For example the water molecule has an molar mass of 18 therefore one mole of water weighs 18 grams. Molecular weight of H2O or mol This compound is also known as Water or Dihydrogen Monoxide. 1 grams H2O is equal to 0. This site explains how to find molar mass. For a given molecule one mole is a mass in grams whose number is equal to the molar mass of the molecule.
 Source: youtube.com
Source: youtube.com
We assume you are converting between grams H2O and mole. The reason is that the molar mass of the substance affects the conversion. In general one mole of any substance contains Avogadros Number of molecules. Using the chemical formula of the compound and the periodic table of elements we can add up the atomic weights and calculate molecular weight of the substance. The SI base unit for amount of substance is the mole.
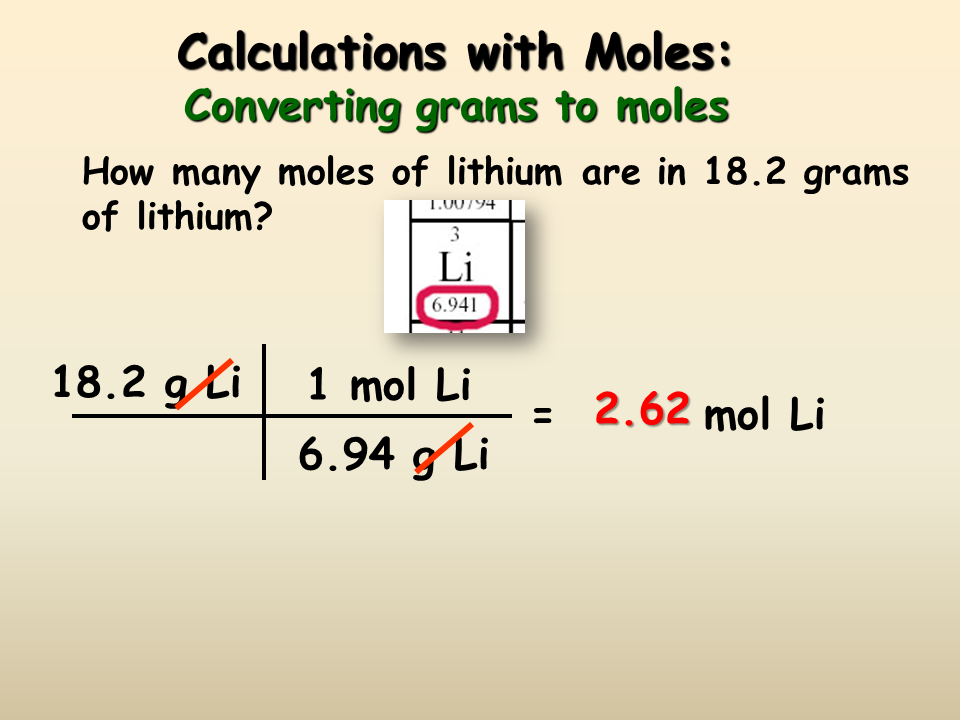
You can view more details on each measurement unit. One lb-mol is equal to 45359237 mol which value is the same as the number of grams in an international avoirdupois pound. We assume you are converting between grams H2O and mole. In the metric system chemical engineers once used the kilogram-mole notation kg-mol which is defined as the number of entities in 12 kg of 12 C and often referred to the mole as the gram-mole notation g-mol when. The reason is that the molar mass of the substance affects the conversion.
 Source: youtube.com
Source: youtube.com
We assume you are converting between grams H2O and mole. For a given molecule one mole is a mass in grams whose number is equal to the molar mass of the molecule. Using the chemical formula of the compound and the periodic table of elements we can add up the atomic weights and calculate molecular weight of the substance. How many grams H2O in 1 mol. For example the water molecule has an molar mass of 18 therefore one mole of water weighs 18 grams.
 Source: youtube.com
Source: youtube.com
Finding molar mass starts with units of grams per mole gmol. One lb-mol is equal to 45359237 mol which value is the same as the number of grams in an international avoirdupois pound. Finding molar mass starts with units of grams per mole gmol. For a given molecule one mole is a mass in grams whose number is equal to the molar mass of the molecule. Similarly a mole of neon has a molar mass of 20 grams.
 Source: youtube.com
Source: youtube.com
Finding molar mass starts with units of grams per mole gmol. You can view more details on each measurement unit. The answer is 1801528. Using the chemical formula of the compound and the periodic table of elements we can add up the atomic weights and calculate molecular weight of the substance. In general one mole of any substance contains Avogadros Number of molecules.
 Source: study.com
Source: study.com
The answer is 1801528. Similarly a mole of neon has a molar mass of 20 grams. Using the chemical formula of the compound and the periodic table of elements we can add up the atomic weights and calculate molecular weight of the substance. This site explains how to find molar mass. In the metric system chemical engineers once used the kilogram-mole notation kg-mol which is defined as the number of entities in 12 kg of 12 C and often referred to the mole as the gram-mole notation g-mol when.
 Source: khanacademy.org
Source: khanacademy.org
The answer is 1801528. Similarly a mole of neon has a molar mass of 20 grams. Using the chemical formula of the compound and the periodic table of elements we can add up the atomic weights and calculate molecular weight of the substance. The reason is that the molar mass of the substance affects the conversion. You can view more details on each measurement unit.
 Source: wikihow.com
Source: wikihow.com
For a given molecule one mole is a mass in grams whose number is equal to the molar mass of the molecule. We assume you are converting between grams H2O and mole. Finding molar mass starts with units of grams per mole gmol. In general one mole of any substance contains Avogadros Number of molecules. The reason is that the molar mass of the substance affects the conversion.
 Source: study.com
Source: study.com
Using the chemical formula of the compound and the periodic table of elements we can add up the atomic weights and calculate molecular weight of the substance. In the metric system chemical engineers once used the kilogram-mole notation kg-mol which is defined as the number of entities in 12 kg of 12 C and often referred to the mole as the gram-mole notation g-mol when. One lb-mol is equal to 45359237 mol which value is the same as the number of grams in an international avoirdupois pound. Finding molar mass starts with units of grams per mole gmol. The SI base unit for amount of substance is the mole.
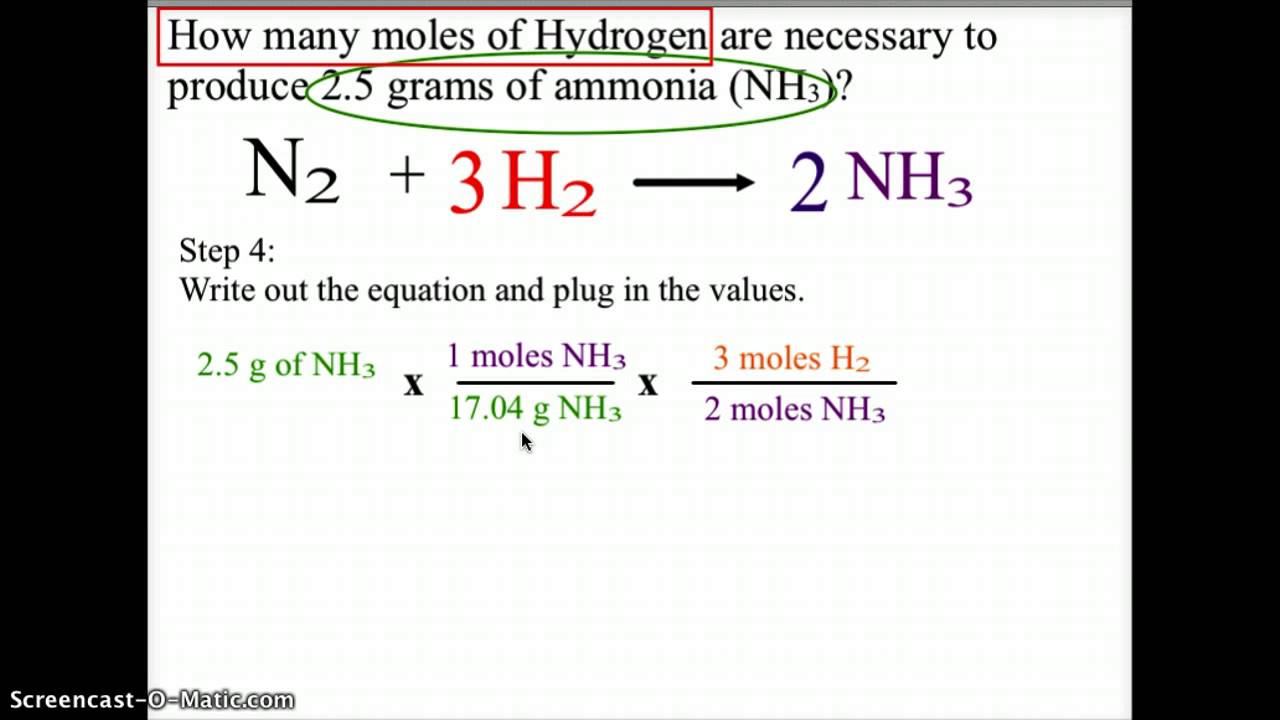 Source: youtube.com
Source: youtube.com
We assume you are converting between grams H2O and mole. Using the chemical formula of the compound and the periodic table of elements we can add up the atomic weights and calculate molecular weight of the substance. Finding molar mass starts with units of grams per mole gmol. In the metric system chemical engineers once used the kilogram-mole notation kg-mol which is defined as the number of entities in 12 kg of 12 C and often referred to the mole as the gram-mole notation g-mol when. For a given molecule one mole is a mass in grams whose number is equal to the molar mass of the molecule.
 Source: youtube.com
Source: youtube.com
We assume you are converting between grams H2O and mole. In general one mole of any substance contains Avogadros Number of molecules. How many grams H2O in 1 mol. Finding molar mass starts with units of grams per mole gmol. For a given molecule one mole is a mass in grams whose number is equal to the molar mass of the molecule.
 Source: youtube.com
Source: youtube.com
The reason is that the molar mass of the substance affects the conversion. One lb-mol is equal to 45359237 mol which value is the same as the number of grams in an international avoirdupois pound. Using the chemical formula of the compound and the periodic table of elements we can add up the atomic weights and calculate molecular weight of the substance. In the metric system chemical engineers once used the kilogram-mole notation kg-mol which is defined as the number of entities in 12 kg of 12 C and often referred to the mole as the gram-mole notation g-mol when. Molecular weight of H2O or mol This compound is also known as Water or Dihydrogen Monoxide.
 Source: clutchprep.com
Source: clutchprep.com
We assume you are converting between grams H2O and mole. Using the chemical formula of the compound and the periodic table of elements we can add up the atomic weights and calculate molecular weight of the substance. The SI base unit for amount of substance is the mole. One lb-mol is equal to 45359237 mol which value is the same as the number of grams in an international avoirdupois pound. For example the water molecule has an molar mass of 18 therefore one mole of water weighs 18 grams.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find grams from mol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






