Your How to find gram molecules of oxygen images are available in this site. How to find gram molecules of oxygen are a topic that is being searched for and liked by netizens now. You can Find and Download the How to find gram molecules of oxygen files here. Find and Download all royalty-free photos and vectors.
If you’re looking for how to find gram molecules of oxygen images information related to the how to find gram molecules of oxygen topic, you have come to the ideal blog. Our site always provides you with hints for downloading the maximum quality video and picture content, please kindly search and find more informative video content and graphics that fit your interests.
How To Find Gram Molecules Of Oxygen. 2 moles Oxygen to grams 319988 grams. In other words 1 mole of oxygen would contain 6023 xx 1023 molecules. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules. Physical Properties Of Oxygen.
 Calculate The Number Of Molecules In 4 G Of Oxygen Youtube From youtube.com
Calculate The Number Of Molecules In 4 G Of Oxygen Youtube From youtube.com
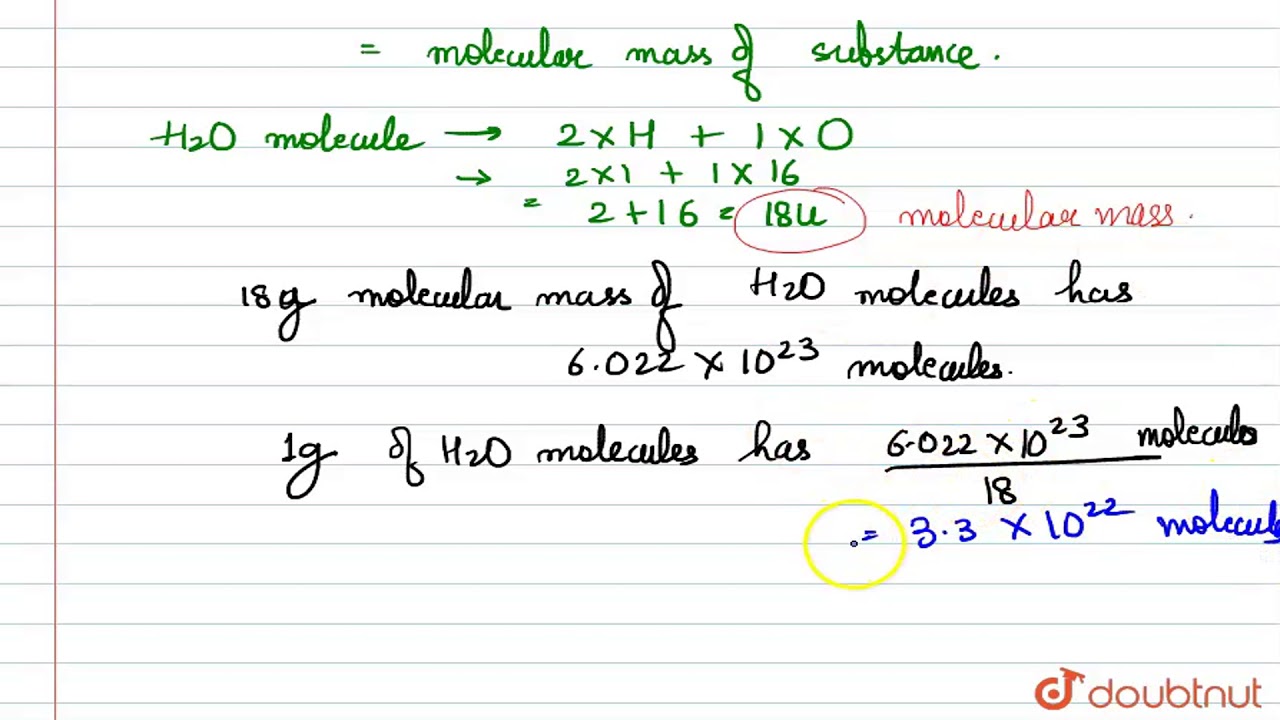
The number of gram molecules of oxygen O 2. Therefore you could say that there are 0015625 moles of atoms in 05g of Oxygen or you could say that there are 0015625 60221023 94093751021 or even 9409375000000000000000000000 atoms in 05g of. Here we are given with 16 g of oxygen. Therefore in 183 g of adrenaline there are 48 g of oxygen. The SI base unit for amount of substance is the mole. 05g of Oxygen 0532 0015625 moles.
Number of gram molecules in 40g of oxygen 1 32 40 1.
05g of Oxygen 0532 0015625 moles. Prev Question Next Question. The molecular formula for Oxygen is O. For one mole atoms of oxygen mass of NO2 will be 23 g and for 4 moles it will be 423 g92 g. Of gram atoms of O60210236021024. Then 6021024COmolecules contain 6021024atoms of O.
 Source: youtube.com
Source: youtube.com
In other words 1 mole of oxygen would contain 6023 xx 1023 molecules. 32g of Oxygen 1 mole. Besides how many moles are in 16 grams of oxygen. 3 moles Oxygen to grams 479982 grams. 05g of Oxygen 0532 0015625 moles.
 Source: youtube.com
Source: youtube.com
7 moles Oxygen to grams 1119958 grams. 6 moles Oxygen to grams 959964 grams. Given mass of oxygen 40g Number of gram atoms in 16g of oxygen 1 gram atom Number of gram atoms in 40g of oxygen 1 16 40 2. 32g of Oxygen 1 mole. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules.

Molecular weight of Oxygen or mol. 4 moles Oxygen to grams 639976 grams. You can view more details on each measurement unit. For example water has a molecular weight of 18015 amu. The molecular formula for Oxygen is O.
 Source: pinterest.com
Source: pinterest.com
2 moles Oxygen to grams 319988 grams. So to summarize the molecular weight will be ideally equal to the mass that one mole of the given substance contains. Therefore in 183 g of adrenaline there are 48 g of oxygen. Of gram atoms of O60210236021024. Please log in or register to add a comment.
 Source: youtube.com
Source: youtube.com
6 moles Oxygen to grams 959964 grams. 4 moles Oxygen to grams 639976 grams. Nitrogen is less soluble in water than oxygen because it has about one molecule of oxygen for every two molecules of nitrogen. The number of gram molecules of oxygen O 2. For example water has a molecular weight of 18015 amu.
 Source: toppr.com
Source: toppr.com
1 grams Oxygen is equal to 0062502343837894 mole. 2 moles of atoms of oxygen are contained in 1 mole of NO2 gas. In other words 1 mole of oxygen would contain molecules. Prev Question Next Question. The number of gram atoms of oxygen in 60210 24 CO molecules is obtained by dividing 60210 24 with Avogadros number.

Nitrogen is less soluble in water than oxygen because it has about one molecule of oxygen for every two molecules of nitrogen. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules. 05g of Oxygen 0532 0015625 moles. Oxygens solubility in water depends on its temperature. Prev Question Next Question.
 Source: toppr.com
Source: toppr.com
Given mass of oxygen 40g Number of gram atoms in 16g of oxygen 1 gram atom Number of gram atoms in 40g of oxygen 1 16 40 2. Quick conversion chart of moles Oxygen to grams. Number of moles 18 g 1008 gmol 2 1600 gmol 18 g 1802 gmol 1 mole. Then 6021024COmolecules contain 6021024atoms of O. Molecular weight of Oxygen or mol.

32g of Oxygen 1 mole. So to summarize the molecular weight will be ideally equal to the mass that one mole of the given substance contains. 2 moles of atoms of oxygen are contained in 1 mole of NO2 gas. In other words 1 mole of oxygen would contain 6023 xx 1023 molecules. Of gm of molecules of oxygen2atomsmolecule10gmatoms 5gmmolecules.
 Source: toppr.com
Source: toppr.com
Prev Question Next Question. Wait a moment and try again. 6 moles Oxygen to grams 959964 grams. Here we are given with 16 g of oxygen. 8 moles Oxygen to.
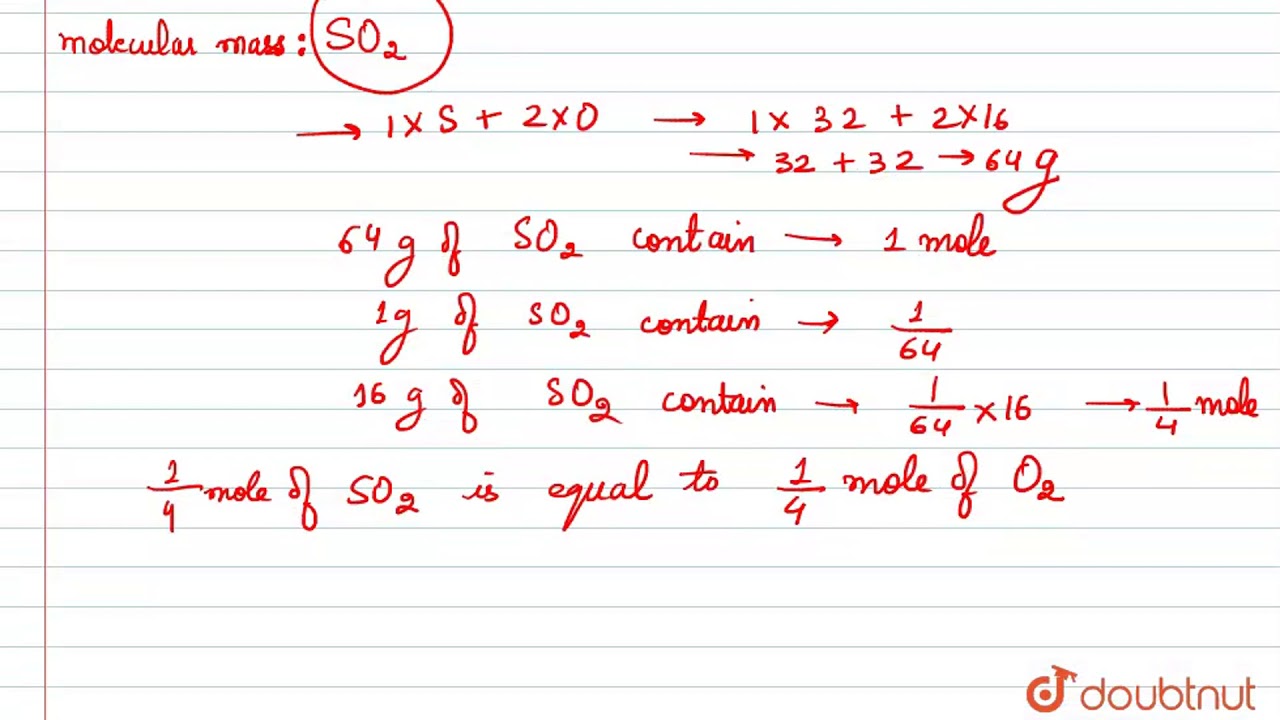 Source: m.youtube.com
Source: m.youtube.com
Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules. Nitrogen is less soluble in water than oxygen because it has about one molecule of oxygen for every two molecules of nitrogen. In other words 1 mole of oxygen would contain molecules. Solve any question of Some Basic Concepts of Chemistrywith-. We also know that 1 m o l of adrenaline weighs 183 g.
 Source: pinterest.com
Source: pinterest.com
In other words 1 mole of oxygen would contain molecules. Given mass of oxygen 40g Number of gram atoms in 16g of oxygen 1 gram atom Number of gram atoms in 40g of oxygen 1 16 40 2. 1 grams Oxygen is equal to 0062502343837894 mole. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules. Therefore the correct option is B.
 Source: youtube.com
Source: youtube.com
32g of Oxygen 1 mole. You can view more details on each measurement unit. The number of molecules in 16 gm of oxygen are 05 1632 moles. That said to find the mass of one ATOM we need to convert from moles to atoms as follows. These numbers are either in atomic mass units amu or in grams per mole of atoms.
 Source: youtube.com
Source: youtube.com
Is equal to one half the number of gram atoms of oxygen. Of gram atoms of O60210236021024. Quick conversion chart of moles Oxygen to grams. 3 moles Oxygen to grams 479982 grams. In other words 1 mole of oxygen would contain molecules.
 Source: pinterest.com
Source: pinterest.com
Besides how many moles are in 16 grams of oxygen. Therefore the percentage composition is 2623. As oxygen is a diatomic molecule. For example oxygens molar weight is approximately 16. In other words 1 mole of oxygen would contain molecules.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to find gram molecules of oxygen by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






