Your How to find gram molecular mass of methane images are ready in this website. How to find gram molecular mass of methane are a topic that is being searched for and liked by netizens now. You can Find and Download the How to find gram molecular mass of methane files here. Get all free images.
If you’re looking for how to find gram molecular mass of methane images information linked to the how to find gram molecular mass of methane keyword, you have visit the right blog. Our website always gives you hints for refferencing the maximum quality video and image content, please kindly hunt and locate more informative video content and graphics that fit your interests.
How To Find Gram Molecular Mass Of Methane. How many moles are in 32 grams of methane. 34 g x 1mol160424g 021 moles C. So its molecular weight is 12 4 16. Note that rounding errors may occur so always check the results.
 Image Result For What Is The Strongest Bond Chemistry Covalent Bonding Study Chemistry Chemical Bond From pinterest.com
Image Result For What Is The Strongest Bond Chemistry Covalent Bonding Study Chemistry Chemical Bond From pinterest.com
How many moles are in methane. Number of moles of methane 35 n. 2SO 4 192 232 16 4 12 H 2 O 216 122 16 Therefore the gram formula mass is 1 mol 474g3. 1 Al 27. The reaction formula CH4 2O2 CO2 2H2O shows the oxidation of 1 mole of CH4 Methane will yield 1 mole of CO2 Carbon Dioxide. Since m - 80g.
M CH4 mole number of CH4 molecular mass m CH4 M CH4 Example.
You can view more details on each measurement unit. Number of moles of sulphur dioxide 24 n. See also our theoretical yield calculator for chemical reactions probably your next stop to finish the problem set. 24 moles of sulphur dioxide. If there is no subscript it means there is only one atom of that element in the molecule. Methane molecular weight.

The reaction formula CH4 2O2 CO2 2H2O shows the oxidation of 1 mole of CH4 Methane will yield 1 mole of CO2 Carbon Dioxide. N m Mm. An amount of any substance in grams that is numerically equal to its atomic or molecular weight in amu has been defined as one mole of that substance. M CH4 mole number of CH4 molecular mass m CH4 M CH4 Example. 2SO 4 192 232 16 4 12 H 2 O 216 122 16 Therefore the gram formula mass is 1 mol 474g3.
 Source: in.pinterest.com
Source: in.pinterest.com
35 moles of methane. 12011 1 12011 u displaystyle. 1 cubic centimeter of methane gas weighs 0000554 gram g 1 cubic inch of methane gas weighs 0000320232 ounce oz Methane gas weighs 0000554 gram per cubic centimeter or 0554 kilogram per cubic meter ie. Number of mole 5moles. What is the molar mass of methane CH4.
 Source: pinterest.com
Source: pinterest.com
N m Mm. M CH4 mole number of CH4 molecular mass m CH4 M CH4 Example. Mass of methane 35 16 56 g. You can view more details on each measurement unit. Number of mole 5moles.
 Source: youtube.com
Source: youtube.com
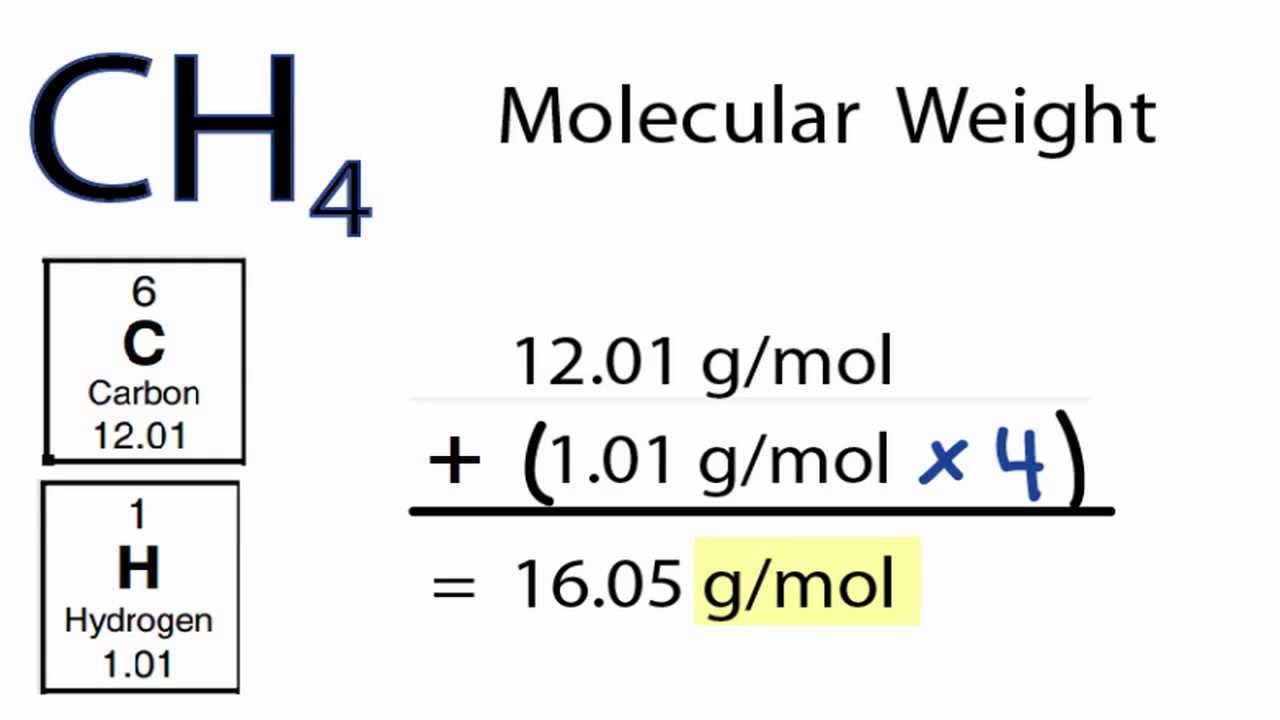
Add together the weight of all the atoms in methane to find the molecular weight. Methane CH4 Methane is a colorless odorless flammable gas that is the simplest hydrocarbon and is the major constituent of natural gas. Add carbon mass with H mass 12 1 x 4 gmol as 4 atoms of H 124 16 gmol. Apr 08 2020 So the overall gram molecular mass might be. The reaction formula CH4 2O2 CO2 2H2O shows the oxidation of 1 mole of CH4 Methane will yield 1 mole of CO2 Carbon Dioxide.
 Source: pinterest.com
Source: pinterest.com
How many moles of methane are in your sample. The molar mass of the methane is M1604gmol M 1604 g m o l. 34 g x 1mol160424g 021 moles C. Density of methane gas is equal to 0554 kgm³. Since 1 mole of CH4 will weigh 12g for the Carbon 4g 1g for each Hydrogen 16g then 32g of CH4 will correspond to 32g 16gmole 2 moles.

If there is no subscript it means there is only one atom of that element in the molecule. To calculate the molar mass add the mass of each element The molecular formula of methane is CH4. 34 g x 1mol160424g 021 moles C. Molecular weight of Methane or grams. Lets calculate the molecular weight of CH4.
 Source: youtube.com
Source: youtube.com
1 Al 27. Grams Moles and Molecular Mass Evaluate 1. Number of moles of methane 35 n. The mass of one gas particle multiplied by Avogadros number is the molecular weight molar mass of the gas 602 x 1023. Knowing an elements or compounds molar mass can assist us in stoichiometrically balancing a reaction equation.
 Source: in.pinterest.com
Source: in.pinterest.com
An amount of any substance in grams that is numerically equal to its atomic or molecular weight in amu has been defined as one mole of that substance. 35 moles of methane. Add together the weight of all the atoms in methane to find the molecular weight. The molar mass of methane is 1605 gmole. The mass of one gas particle multiplied by Avogadros number is the molecular weight molar mass of the gas 602 x 1023.
 Source: pinterest.com
Source: pinterest.com
Add all of the values together to find the gram molecular mass. So gram weight 093 16043 1491999 gram. One carbon atom 4 hydrogen atoms 120108 410079 160424 gmol B. There are 4 hydrogen atoms in methane. Mm - molar mass.
 Source: pinterest.com
Source: pinterest.com
1008 4 4032 u displaystyle 100844032u. Molecular mass of methane CH 4 12 x 1 1 x 4 12 4 16 g. Lets calculate the molecular weight of CH4. How many moles are in methane. To find the number of mole of methanewe will use the formula.
 Source: pinterest.com
Source: pinterest.com
Mm - molar mass. Number of moles of sulphur dioxide 24 n. So the total gram molecular mass will be. What is the molar mass of methane. The molecular formula for Methane is CH4.
 Source: youtube.com
Source: youtube.com
Grams Moles and Molecular Mass Evaluate 1. How many moles are in methane. You can view more details on each measurement unit. So 32g of methane will be 2 moles of it. There are 4 hydrogen atoms in methane.
 Source: youtube.com
Source: youtube.com
42 12 31 64 149 gmol. The molar mass of methane is 1605 gmole. Therefore molar mass of methane 4 1 12 16 grams. Molecular mass of hydrogen 1 gram. Molecular weight of CH4 M CH4 16043.
 Source: pinterest.com
Source: pinterest.com
The molecular formula for Methane is CH4. So 32g of methane will be 2 moles of it. Number of moles can be. The molar mass of CH4 methane is. At 0C 32F or 27315K at standard atmospheric pressure.
 Source: pinterest.com
Source: pinterest.com
How many moles of methane are in your sample. How many moles are in 32 grams of methane. Since 1 mole of CH4 will weigh 12g for the Carbon 4g 1g for each Hydrogen 16g then 32g of CH4 will correspond to 32g 16gmole 2 moles. First find the number of moles of methane and number of moles of oxygen using the formula- Number of molesdfractextGiven masstextMolecular mass. Add carbon mass with H mass 12 1 x 4 gmol as 4 atoms of H 124 16 gmol.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to find gram molecular mass of methane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






