Your How to find g mol in chemistry images are ready in this website. How to find g mol in chemistry are a topic that is being searched for and liked by netizens today. You can Get the How to find g mol in chemistry files here. Find and Download all royalty-free vectors.
If you’re searching for how to find g mol in chemistry pictures information linked to the how to find g mol in chemistry interest, you have pay a visit to the right site. Our website always gives you suggestions for seeking the highest quality video and picture content, please kindly surf and locate more informative video articles and graphics that fit your interests.
How To Find G Mol In Chemistry. Amount mass molar mass. So just to be sure. For example 100 g of water is about 5551 mol of water. μ rms 3RTM ½.
 Chemistry Mole Conversion Chart Images Pictures Becuo Chemistry Education Chemistry Lessons Teaching Chemistry From br.pinterest.com
Chemistry Mole Conversion Chart Images Pictures Becuo Chemistry Education Chemistry Lessons Teaching Chemistry From br.pinterest.com
The number of moles of a substance in a sample is obtained by dividing the mass of the sample by the molar mass of the compound. For hydrogen chloride the molar mass is 1007 35453 36460 gmol. In this case there is only one sodium Na atom and chlorine Cl atom in. The second way to calculate Δ G is to use a formula that involves enthalpy temperature and entropy. This is the weight in grams of 1 mole of the compound. Δ G is in the units Joules J.
Given a samples mass and number of moles in that sample it is also possible to calculate the samples molecular mass by dividing the mass by the number of moles to calculate gmol.
I think Im missing. Convert the volume of the water to its mass assuming that the density of pure water is 998 kgm³. For hydrogen chloride the molar mass is 1007 35453 36460 gmol. This can be rearranged to find the mass if the number of moles and molar mass its relative formula mass in grams are known. Other methods include the use of the molar volume or the measurement of electric charge. First molar mass of NaCl should be calculated.
 Source: pinterest.com
Source: pinterest.com
So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl. Therefore NaCl 584538 gL. Its easier to work with grams so. We receive this kind of Molecular Weight To Moles graphic could possibly be the most trending topic taking into account we part it in google improvement or facebook. First molar mass of NaCl should be calculated.
 Source: pinterest.com
Source: pinterest.com
Δ G is in the units Joules J. For hydrogen chloride the molar mass is 1007 35453 36460 gmol. Therefore NaCl 584538 gL. I think Im missing. T is in the units of Kelvin K.
 Source: pinterest.com
Source: pinterest.com
Molar mass of NaCl 585 g mol-1. The average velocity of gas particles is found using the root mean square velocity formula. μ rms 3RTM ½. Its submitted by handing out in the best field. Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom.
 Source: es.pinterest.com
Source: es.pinterest.com
The second way to calculate Δ G is to use a formula that involves enthalpy temperature and entropy. 0700 mole x 340146 gramsmole 238 grams The answer of 238 g has been rounded to three significant figures because the 0700 value had the least number of significant figures in the problem. First molar mass of NaCl should be calculated. Example If you want to find the molar mass of common salt Sodium Chloride- NaCl You add the mass of each element of it. Δ H is in the units of Joules J.
 Source: pinterest.com
Source: pinterest.com
Now we can find the NaCl amount using mass molar mass and amount relationship. So a mole of water H 2 O has a mass of 18 g. Other methods include the use of the molar volume or the measurement of electric charge. The average velocity of gas particles is found using the root mean square velocity formula. μ rms root mean square velocity in msec.
 Source: br.pinterest.com
Source: br.pinterest.com
Add the weight of each atom in the compound. M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988. To find the molar mass of sodium chloride you first go to the periodic table. Lets calculate the molar mass of NaCl. Δ G is in the units Joules J.
 Source: pinterest.com
Source: pinterest.com
0170 moles Ni 60221023 atoms Ni 1 mol Ni 1021023 atoms Ni 0170 moles Ni 6022 10 23 atoms Ni 1 mol Ni 102 10 23 atoms Ni. Therefore NaCl 584538 gL. We identified it from well-behaved source. μ rms root mean square velocity in msec. The number of moles of a substance in a sample is obtained by dividing the mass of the sample by the molar mass of the compound.
 Source: pinterest.com
Source: pinterest.com
If you have a pure liquid or a solid you use its density to calculate its mass and then divide the mass by the molar mass. Find the molar mass of a water molecule H₂O. Other methods include the use of the molar volume or the measurement of electric charge. For example the molar mass of water is 18015 gmol. I know I should divide g L 1 by the molar mass of the substance but I dont seem to find the specific answer on Google.
 Source: pinterest.com
Source: pinterest.com
A mole of carbon dioxide CO 2 has a mass of 44 gThis also works for ionic compounds so a mole of sodium chloride NaCl has a mass of 585 g. First find the molar mass of NaCl. In this case there is only one sodium Na atom and chlorine Cl atom in. Multiply the moles given by the substances molar mass. Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom.
 Source: pinterest.com
Source: pinterest.com
So just to be sure. Mass of dissolved NaCl is given. In this case ethanols molecular mass is 46 gmol. In this case there is only one sodium Na atom and chlorine Cl atom in. 1 Gram-mole g-mol 1 000 Millimole mmol - Measurement calculator that can be.
 Source: pinterest.com
Source: pinterest.com
For glucose the molar mass is 720642. It can also be. We identified it from well-behaved source. I know I should divide g L 1 by the molar mass of the substance but I dont seem to find the specific answer on Google. Calculate Molecular Weight - 9 images - molar mass molecular weight of ca oh 2 calcium 1 find the formula mass of c3h5o2 3 g 5.
 Source: pinterest.com
Source: pinterest.com
This is a lot easier than counting out all those molecules. In this case ethanols molecular mass is 46 gmol. Furthermore how do you convert g mol to mmol. This is a lot easier than counting out all those molecules. Find the molar mass of a water molecule H₂O.
 Source: pinterest.com
Source: pinterest.com
For example the molar mass of water is 18015 gmol. The number of moles of a substance in a sample is obtained by dividing the mass of the sample by the molar mass of the compound. This can be rearranged to find the mass if the number of moles and molar mass its relative formula mass in grams are known. Molar mass of NaCl 23 1 3551. For glucose the molar mass is 720642.
 Source: pinterest.com
Source: pinterest.com
The formula is below. However again because all of these are linked to chemistry and because chemistry likes to measure. Δ G is in the units Joules J. Δ H is in the units of Joules J. If I have 10 5 g L 1 C u X 2 solution do I have 157 10 7 m o l L 1.
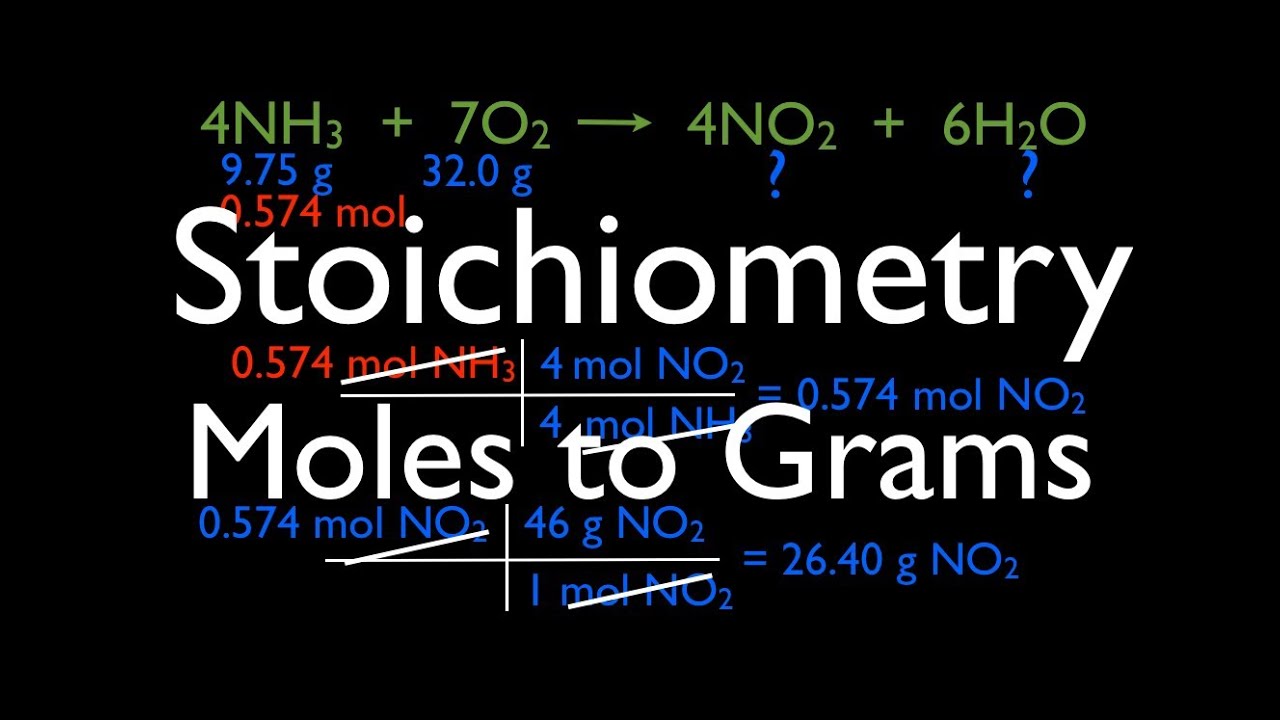 Source: pinterest.com
Source: pinterest.com
It can also be. 0170 moles Ni 60221023 atoms Ni 1 mol Ni 1021023 atoms Ni 0170 moles Ni 6022 10 23 atoms Ni 1 mol Ni 102 10 23 atoms Ni. It can also be. For hydrogen chloride the molar mass is 1007 35453 36460 gmol. Multiply the moles given by the substances molar mass.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find g mol in chemistry by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






