Your How to find 1 mole of carbon images are available in this site. How to find 1 mole of carbon are a topic that is being searched for and liked by netizens now. You can Download the How to find 1 mole of carbon files here. Find and Download all free photos and vectors.
If you’re looking for how to find 1 mole of carbon pictures information linked to the how to find 1 mole of carbon interest, you have visit the right site. Our website frequently provides you with hints for seeing the highest quality video and picture content, please kindly search and locate more informative video articles and images that match your interests.
How To Find 1 Mole Of Carbon. You will need per liter ceKOH. This number is known as Avogadros number. And you are quoted molar masses of EVERY chemical element on the Periodic Table. Environment Canada publishes factors to estimate CO2 and.
 If One Mole Of Carbon Atom S Weighs 12 Grams What Is Mass In Grams Of 1 Atom Of Carbon Youtube From youtube.com
If One Mole Of Carbon Atom S Weighs 12 Grams What Is Mass In Grams Of 1 Atom Of Carbon Youtube From youtube.com
Molecular mass of carbon 12. Molecular weight of carbon or mol The molecular formula for carbon is C. Use this page to learn how to convert between grams of carbon and mole. 1 grams of carbon is equal to 0083259093974539 mole. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl. The SI base unit for amount of substance is the mole.
I think you want the number of moles of positive ions so this will be moles of NH4 1811022 x 5 there are 5 atoms in NH4 9061022 Nmber of NH4 ions 181 x 10 22 ions and the number of moles of NH4 ions 00301 moles.
OK how much carbon dioxide is needed to transform all hydroxide ions into potassium hydrocarbonate. Thus one mole of carbon dioxide gas is equal to one mole of oxygen. Molecular mass of carbon 12. Avagadros Law states that equal number of molecules of any gas will occupy the same volume. Since the molecular mass of carbon-12 is 12 there are 12 grams in 1 mole of it and therefore 2012 53 moles in 20 grams. 1 mole of carbon.
 Source: youtube.com
Source: youtube.com
Use this page to learn how to convert between grams of carbon and mole. One mole is defined as the amount of substance that contains as many particles as the number of atoms in exactly 12 g of carbon-12 which is 602 10 23 particles. One mole of carbon dioxide will occupy 245 L at RTP. The number of atoms of carbon-12 present in this one mole sample is 6022 136 7 x 10 23. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl.
 Source: toppr.com
Source: toppr.com
This is defined as 0001 kilogram per mole or 1 gram per mole. You will need per liter ceKOH. Note that rounding errors may occur so always check the results. Environment Canada publishes factors to estimate CO2 and. After addition of pu016 mol carbon dioxide we will face the same situation as if you had dissolved pu016 mol of potassium hydrogencarbonate in pu1 L of water.

Third find of positive charge atoms. One mole of H 2 O molecules contains 6022 x 10 23 molecules. After addition of pu016 mol carbon dioxide we will face the same situation as if you had dissolved pu016 mol of potassium hydrogencarbonate in pu1 L of water. From compound to compound to a number of atoms in a molecule vary. At 25C 77F or 29815K at standard atmospheric pressure.

1 mole 60221023 so 44 amu73607610-33 grams approx. 10-4 kg of carbon dioxide. The amount of material counting 602214 1023 particles The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. I think you want the number of moles of positive ions so this will be moles of NH4 1811022 x 5 there are 5 atoms in NH4 9061022 Nmber of NH4 ions 181 x 10 22 ions and the number of moles of NH4 ions 00301 moles. 1 mole is equal to 1 moles CO2 or 440095 grams.
 Source: toppr.com
Source: toppr.com
This converts atomic units to grams per mole making the molar mass of hydrogen 1007 grams per mole of. This converts atomic units to grams per mole making the molar mass of hydrogen 1007 grams per mole of. Thus one mole of carbon dioxide gas is equal to one mole of oxygen. Avagadros Law states that equal number of molecules of any gas will occupy the same volume. One mole of carbon dioxide will occupy 245 L at RTP.
 Source: slideplayer.com
Source: slideplayer.com
Second you need to know how many moles there are in 20 grams of carbon-12. And you are quoted molar masses of EVERY chemical element on the Periodic Table. Which gives more CO2 per gram fuel. The amount of a substance that contains the same number of entities as there are atoms in 12 g of carbon-12. Molecular weight of carbon or mol The molecular formula for carbon is C.
 Source: teachoo.com
Source: teachoo.com
So 1 mole of sucrose contains 12 moles of carbon atoms 22 moles of hydrogen atoms and 11 moles of oxygen atoms. Avagadros Law states that equal number of molecules of any gas will occupy the same volume. Exactly 12 g of carbon-12 contains 6022 x 10 23 atoms. Third find of positive charge atoms. From compound to compound to a number of atoms in a molecule vary.
 Source: brainly.in
Source: brainly.in
So 1 mole of sucrose contains 12 moles of carbon atoms 22 moles of hydrogen atoms and 11 moles of oxygen atoms. The amount of a substance that contains the same number of entities as there are atoms in 12 g of carbon-12. The number of atoms of carbon-12 present in this one mole sample is 6022 136 7 x 10 23. Molecular mass of CO 2 12 1 16 2 12 32 44 g. 1 mole 60221023 so 44 amu73607610-33 grams approx.
 Source: clutchprep.com
Source: clutchprep.com
After addition of pu016 mol carbon dioxide we will face the same situation as if you had dissolved pu016 mol of potassium hydrogencarbonate in pu1 L of water. The subscripts following each element symbol indicate the number of atoms of each element in the molecule. Use this page to learn how to convert between grams of carbon and mole. One mole is defined as the amount of substance that contains as many particles as the number of atoms in exactly 12 g of carbon-12 which is 602 10 23 particles. Since the molecular mass of carbon-12 is 12 there are 12 grams in 1 mole of it and therefore 2012 53 moles in 20 grams.
 Source: youtube.com
Source: youtube.com
1 grams of carbon is equal to 0083259093974539 mole. Density of carbon dioxide is equal to 1836 kgm³. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. Second you need to know how many moles there are in 20 grams of carbon-12. Third find of positive charge atoms.
 Source: youtube.com
Source: youtube.com
Do a quick conversion. Molecular mass of oxygen 16. Therefore NaCl 584538 gL. Since the molecular mass of carbon-12 is 12 there are 12 grams in 1 mole of it and therefore 2012 53 moles in 20 grams. This number is known as Avogadros number.
 Source: chemistry.wustl.edu
Source: chemistry.wustl.edu
For one mole CO2 73607610-3360221023 which is approximately equals to 44 grams. 1 Mole Of Carbon Has A Mass Of. One mole is defined as the amount of substance that contains as many particles as the number of atoms in exactly 12 g of carbon-12 which is 602 10 23 particles. NaCl 229898 gL 354530 gL. 1 mole is equal to 1 moles CO2 or 440095 grams.
 Source: chemistry.wustl.edu
Source: chemistry.wustl.edu
Exactly 12 grams of pure carbon-12 powder is known as one mole. The amount of a substance that contains the same number of entities as there are atoms in 12 g of carbon-12. Thus one mole of carbon dioxide gas is equal to one mole of oxygen. The amount of material counting 602214 1023 particles The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. Number of moles of CO 2 1136 10-2 and Number of molecules 6843 10 21.
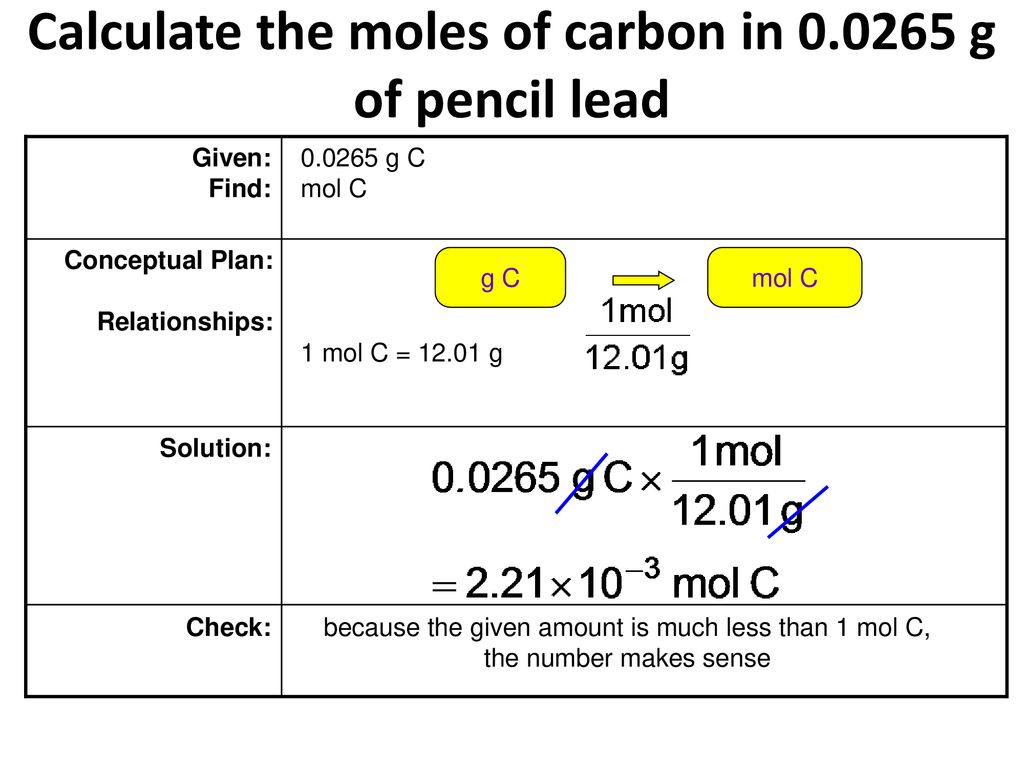 Source: slideplayer.com
Source: slideplayer.com
Since the molecular mass of carbon-12 is 12 there are 12 grams in 1 mole of it and therefore 2012 53 moles in 20 grams. NaCl 229898 gL 354530 gL. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. 1 mole is equal to 1 moles CO2 or 440095 grams. The amount of material counting 602214 1023 particles The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12.
 Source: youtube.com
Source: youtube.com
Second you need to know how many moles there are in 20 grams of carbon-12. Since the molecular mass of carbon-12 is 12 there are 12 grams in 1 mole of it and therefore 2012 53 moles in 20 grams. 1 mole 60221023 so 44 amu73607610-33 grams approx. This converts atomic units to grams per mole making the molar mass of hydrogen 1007 grams per mole of. Question From - NCERT Chemistry Class 9 Chapter 03 Question 013 ATOMS AND MOLECULES CBSE RBSE UP MP BIHAR BOARDQUESTION TEXT-If one mole of carbon at.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to find 1 mole of carbon by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






